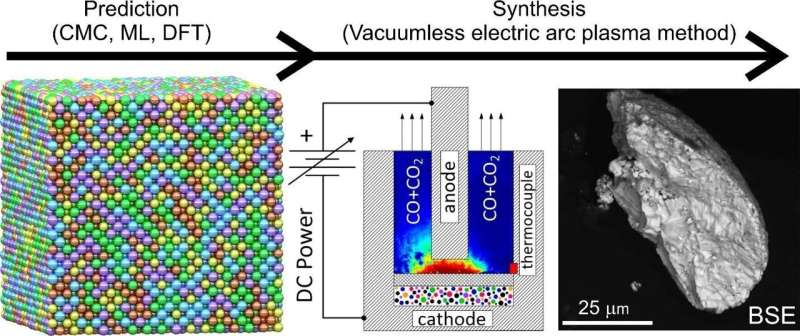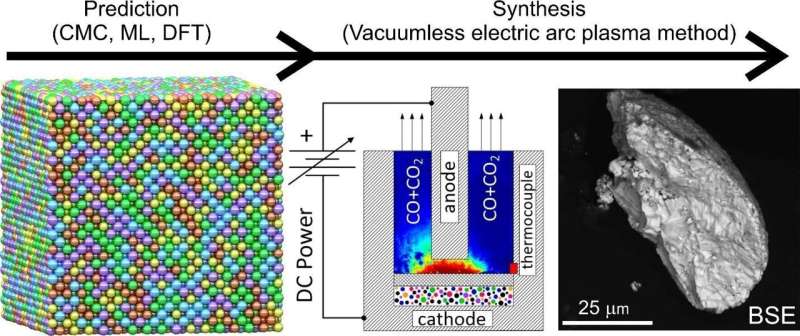
Researchers from Skoltech and Tomsk Polytechnic University have tuned the synthesis of a five-element carbide—a strong, hard-melting compound of carbon and five transition metals—which holds much promise for industrial ceramics and catalysis.
The team relied on fundamental theoretical principles, simulations, and machine learning to identify conditions for the synthesis of single-phase carbide, in which all metal atoms are evenly distributed throughout the crystal. The predictions were confirmed by an experiment using the advanced energy-efficient vacuumless electric arc synthesis method. The study is published in npj Computational Materials.
High-entropy carbides (HECs) are multicomponent equimolar single-phase solid solutions of five or more metals from groups four and five of the periodic table with a cubic NaCl-type crystal structure. One of the key factors of HEC stabilization is configurational, or mixing, entropy, which should be higher than 1.5 times the universal gas constant. This means that the compound should contain at least five basic elements.
HECs attract increasing attention from researchers owing to their unique mechanical properties, a high melting point, and low thermal conductivity. Also, they boast greater hardness, fracture toughness, and temperature stability than individual metal carbides.
The most common HEC synthesis method is reactive spark plasma sintering (SPS) of pre-homogenized raw materials based on individual metal carbides, pure metals, or metal oxides. Typically, HECs are synthesized at high temperatures of about 2,200-2,300 degrees Celsius, and SPS is applied at pressures of about 10–60 MPa with a dwell of 10–15 minutes, whereas homogenization of raw materials can take more than one day.
“The vacuumless electric arc synthesis method helps to produce powdered HECs without using complex, expensive and energy-consuming equipment, such as vacuum pumps for high vacuum or compressors for external pressure. Our set-up is unique in that it does not need pressure or vacuum to produce HECs,” Alexander Pak, the head of the Advanced Energy Materials Laboratory and manager of the Energy of the Future strategic project at TPU, explains.
“Moreover, we can obtain both multi-phase and single-phase carbides by flexibly varying synthesis parameters. HECs are a fairly new class of materials, so we have yet to identify their actual properties and potential uses, which will likely include refractory ceramics and various types of catalysts.”
The team used the Canonical Monte Carlo (CMC) simulation with machine learning interatomic potentials and performed first-principles calculations to determine synthesis temperatures for single- and multiphase carbides. According to the calculations, low-temperature synthesis will produce mostly multiphase HECs with two or more coexisting phases of multicomponent carbides, while synthesis at above 1,500 C will result in single-phase HECs.
“By applying the CMC method, we have succeeded in predicting the HEC’s thermodynamically stable crystal structure at different synthesis temperatures and, as a result, finding the multi-to-single phase transition temperature. Our experiments at temperatures below and above the transition point confirmed the simulation results. Thus, we were able to implement a complete research sequence, going all the way from a computer model to a physical sample,” Skoltech Ph.D. student Vadim Sotskov from the AI for materials science group notes.
More information: Alexander Ya. Pak et al, Machine learning-driven synthesis of TiZrNbHfTaC5 high-entropy carbide, npj Computational Materials (2023). DOI: 10.1038/s41524-022-00955-9
Provided by Skolkovo Institute of Science and Technology