
Chemists at Hokkaido University and the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) have developed the first high-performance catalyst specifically designed and optimized for solid-state, mechanochemical synthesis.
The team found that by attaching long polymer molecules to a metal catalyst, they could trap the catalyst in a fluid-phase, which enabled efficient reactivity at near room temperature. This approach, reported in the Journal of the American Chemical Society, could bring cost and energy savings if adapted for wide application in chemical research and industry.
Chemical synthetic reactions are usually performed in solution, where dissolved molecules can intermingle and react freely. In recent years, however, chemists have developed a process called mechanochemical synthesis, in which solid state crystals and powders are ground together. This approach is advantageous because it reduces the use of hazardous solvents and can allow reactions to proceed faster and at lower temperatures, saving energy costs. It can also be used for reactions between compounds that are difficult to dissolve in available solvents.
However, solid-state reactions occur in a very different environment than solution-based reactions. Previous studies found that palladium complex catalysts originally designed for use in solution often did not work sufficiently in solid-state mechanochemical reactions, and that high reaction temperatures were required. Using the unmodified palladium catalyst for solid-state reactions resulted in limited efficiency due to the tendency of palladium to aggregate into an inactive state. The team chose to embark in a new direction, designing a catalyst to overcome this mechanochemical problem of aggregation.
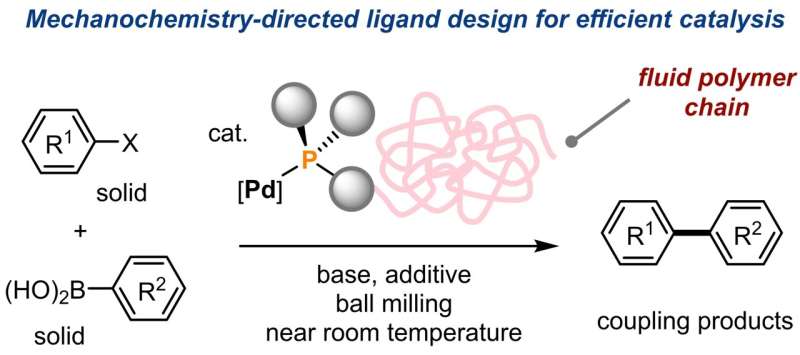
“We developed an innovative solution, linking palladium through a specially designed phosphine ligand to a large polymer molecule called polyethylene glycol,” researcher Hajime Ito explains.
The polyethylene glycol molecules form a region between the solid materials that behaves like a molecular-level fluid phase, where mechanochemical Suzuki-Miyaura cross-coupling reactions proceed much more efficiently and without the problematic aggregation of palladium. In addition to achieving significantly higher product yields, the reaction proceeded effectively near room temperature—the previously best-performing alternative required heating to 120°C. Similar cross-coupling reactions are widely used in research and the chemical industry.
“This is the first demonstration of a system that is specifically modified to harness the potential of palladium complex catalysts in the unique environment of a mechanochemical reaction,” says researcher Koji Kubota.
They believe it could be adapted for many other reactions, and also for catalysts using other elements from the transition metals of the periodic table.
The wider adoption of the process, and others like it, could eventually bring significant savings in costs and energy consumption in commercial chemical processes while allowing more environmentally friendly large-scale production of many useful chemicals.
More information: Tamae Seo et al, Mechanochemistry-Directed Ligand Design: Development of a High-Performance Phosphine Ligand for Palladium-Catalyzed Mechanochemical Organoboron Cross-Coupling, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.2c13543
Journal information: Journal of the American Chemical Society
Provided by Hokkaido University

