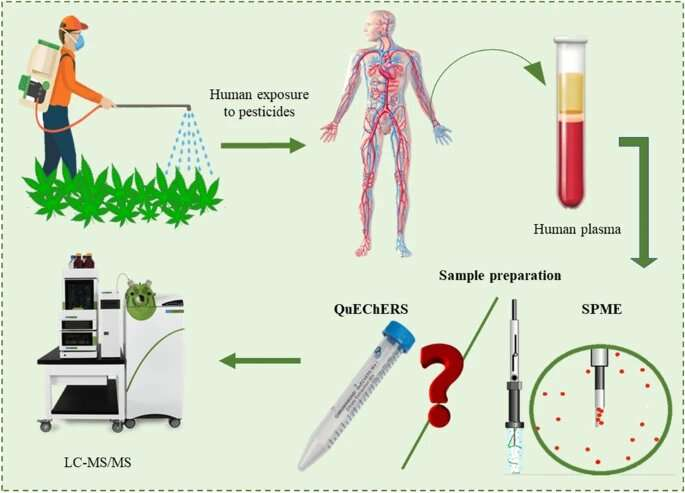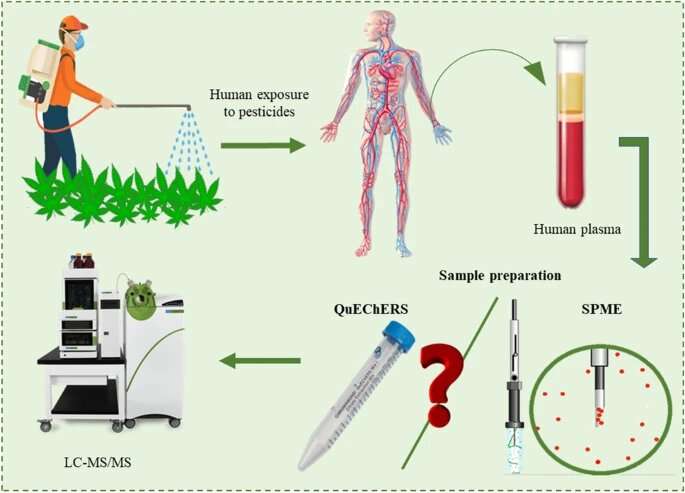
A chemical analyst and expert in micro-extraction at The University of Toledo created a more reliable, robust and efficient way to monitor pesticide exposure and help protect the health and safety of agricultural workers, especially for emerging sectors like the cannabis industry.
Dr. Emanuela Gionfriddo, an assistant professor of analytical chemistry, and Nipunika H. Godage, a Ph.D. candidate in UToledo’s Dr. Nina McClelland Laboratory for Water Chemistry and Environmental Analysis, published research in the journal Analytical and Bioanalytical Chemistry outlining their groundbreaking method that is able to detect 79 pesticide residues in human blood plasma at “ultra-trace” levels, or parts per trillion.
“This has the potential to be applied to human exposure studies for the general public such as exposure through food or contaminated water but, most importantly, agricultural workers who have a higher potential for acute exposure to these toxic chemicals, which typically occurs through the skin, with pesticides then passing into the bloodstream and circulating through the body,” Gionfriddo said.
Pesticides are widely used in farming to prevent or reduce produce losses caused by pests and improve the quality of fruits and vegetables, but human exposure during mixing or application has been reported to cause neurological disorders, poisoning, cancer, reproductive disruptions, respiratory problems and chronic kidney diseases among farm workers.
Though pesticides are regulated by the U.S. Environmental Protection Agency, Gionfriddo said the legalization of cannabis recently in several states has led to “inexperienced” farmers being exposed to the harmful chemicals since those workers are less familiar with pesticide safety equipment and procedures as well as proper pesticide storage and handling.
The pesticides selected for her study are the most commonly used pesticides during cannabis cultivation.
Gionfriddo’s new testing method uses what’s called bio solid-phase microextraction with liquid chromatography-tandem mass spectrometry.
“To meet the growing demands of regulatory agencies and routine analysis laboratories, sample throughput and method tunability is critical,” Gionfriddo said. “Using automated samplers, the preparation time per sample is 1.7 minutes.”
And as occupational exposure to pesticides can occur at varying concentration levels, it is important for any method to quantify pesticides at low concentrations. The new testing method demonstrated higher sensitivity, precision and accuracy and a drastic reduction in abnormalities compared to the commonly used approach, known as QuEChERS, which stands for Quick, Easy, Cheap, Effective, Rugged and Safe but can be labor intensive with prolonged workflows.
Last week during National Farmworker Awareness Week, the U.S. EPA said pesticide exposure doesn’t only happen when working in the fields. The federal agency said pesticide take-home exposure can occur when farm workers go home bearing pesticide residues that may cling to their skin, clothing, hats, boots, tools, lunch coolers or other items in their work environment. Their children may then be exposed to these pesticide residues.
“Assessing pesticide exposure quickly and thoroughly is crucial for the health and safety of workers and their families, to correct malpractices in pesticide storage and application, and to prevent further exposure,” Godage said. “Our new method can extract and analyze simultaneously a wide variety of pesticides from human plasma.”
More information: Nipunika H. Godage et al, Quantitative determination of pesticides in human plasma using bio-SPME-LC–MS/MS: a robust tool to assess occupational exposure to pesticides, Analytical and Bioanalytical Chemistry (2023). DOI: 10.1007/s00216-023-04589-8
Journal information: Analytical and Bioanalytical Chemistry
Provided by University of Toledo