A study led by Dr. Wei Chen, Prof. Yuqin Zou, and Prof. Shuangyin Wang (State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Advanced Catalytic Engineering Research Center of the Ministry of Education, Hunan University) unravels the reaction mechanism of the primary alcohol/vicinal diol electrooxidation reaction on NiO, especially for the synergy between the electrochemical and non-electrochemical steps.
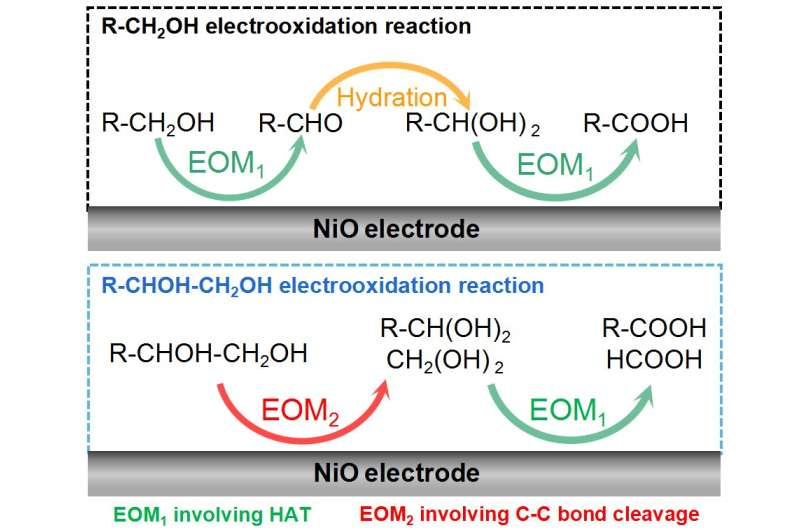
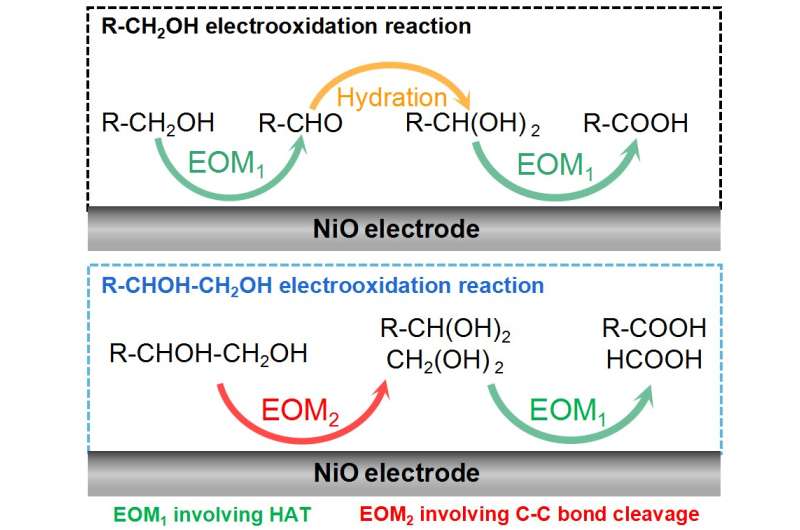
The alcohol electrooxidation on NiO is an indirect electrooxidation reaction with the electrophilic oxygen species as the redox mediator. Therefore, the electrochemical step is the electrochemical generation of electrophilic oxygen species. The research team found two kinds of NiO electrocatalyst function mechanisms, i.e., the electrophilic oxygen-mediated mechanism involving hydrogen atom transfer (EOM-HAT) and the electrophilic oxygen-mediated mechanism involving C-C bond cleavage (EOM-Cleavage of C-C bond).
The synergy between EOM-HAT and the hydration of aldehyde results in the electrooxidation of primary alcohol (R-CH2OH) to carboxylic acid (R-COOH) on NiO. On the other hand, the synergy between EOM-HAT and EOM-Cleavage of the C-C bond causes the electrooxidation of vicinal diol (R-CHOH-CH2OH) to R-COOH and formic acid (HCOOH) on NiO.
This study, published in the journal National Science Review, highlights the synergy between the electrochemical and non-electrochemical steps in the alcohol electrooxidation reaction. It establishes a unified reaction mechanism of alcohol electrooxidation reaction based on nickel-based electrocatalysts.
More information: Wei Chen et al, Unraveling the electrophilic oxygen-mediated mechanism for alcohol electrooxidation on NiO, National Science Review (2023). DOI: 10.1093/nsr/nwad099
Provided by Science China Press