by JooHyeon Heo, Ulsan National Institute of Science and Technology
A research team, led by Professor Ja Hyoung Ryu from the Department of Chemistry at UNIST, in collaboration with Professor Hyewon Chung from Konkuk University, has achieved a significant breakthrough in the treatment of age-related diseases. Their cutting-edge technology offers a promising new approach by selectively removing aging cells, without harming normal healthy cells. This groundbreaking development is poised to redefine the future of health care and usher in a new era of targeted therapeutic interventions.
Aging cells, known as senescent cells, contribute to various inflammatory conditions and age-related ailments as humans age. To address this issue, the research team focused on developing a technology that could precisely target and eliminate aging cells, while sparing normal healthy cells.
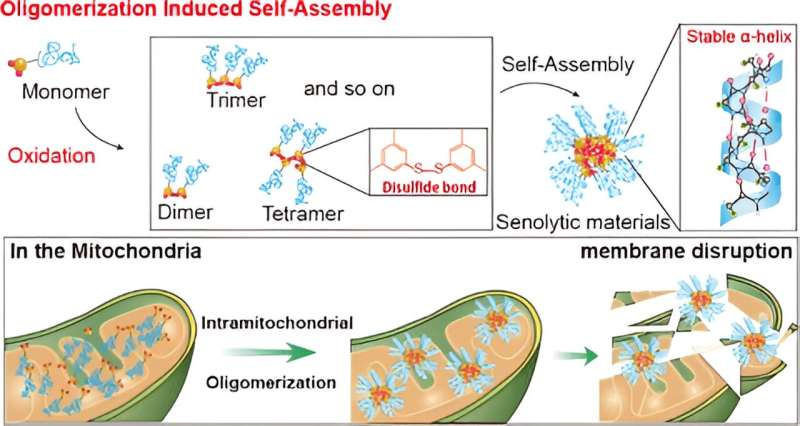
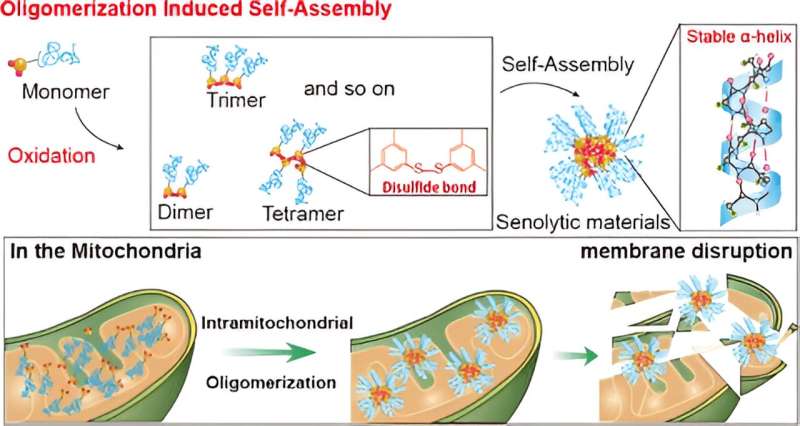
In their study, the team designed organic molecules that selectively target receptors overexpressed in the membranes of aging cells. By leveraging the higher levels of reactive oxygen species (ROS) found in aging cells, these molecules promote the formation of disulfide bonds and create oligomers that bind together. The research is published in the Journal of the American Chemical Society.
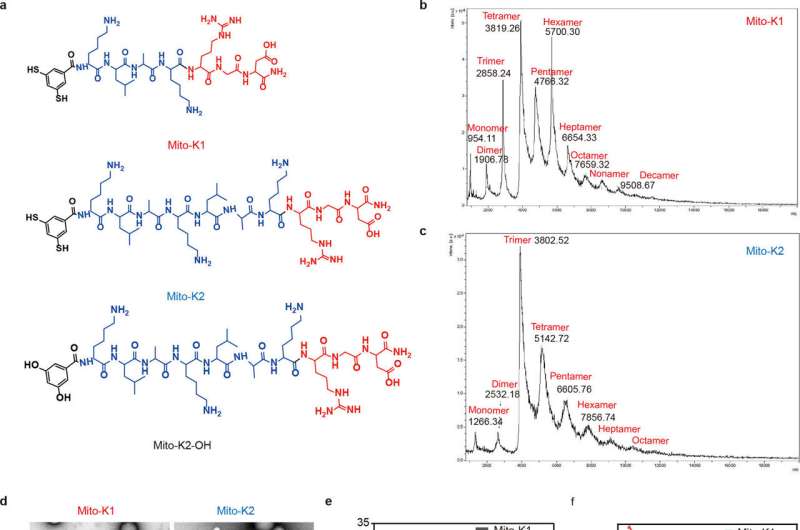
Through self-assembly of these oligomers, the researchers successfully created artificial proteins with a stable α-helix secondary structure. These protein-like nanoassemblies exhibited strong binding affinity to the mitochondrial membranes of aging cells, leading to membrane disruption and subsequent cell self-destruction.
“The selective removal of aging cells by targeting the mitochondria and inducing dysfunction has been successfully demonstrated in our experiments,” stated Professor Ryu. “This approach represents a new paradigm for treating age-related diseases.”
This innovative technology offers several advantages, including minimal toxicity concerns and a wide therapeutic window by specifically targeting organelles within cells. It opens up exciting possibilities for designing preclinical and clinical trials in the future.
More information: Sangpil Kim et al, Supramolecular Senolytics via Intracellular Oligomerization of Peptides in Response to Elevated Reactive Oxygen Species Levels in Aging Cells, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c06898
Journal information: Journal of the American Chemical Society
Provided by Ulsan National Institute of Science and Technology