A group of researchers have unraveled the mysteries behind a recently identified material—zirconium nitride (ZrN)—that helps power clean energy reactions. Their proposed framework will help future designs for transition metal nitrides, paving a path for generating cleaner energy.
The study was published in the journal Chemical Science on July 26, 2023, where it featured as the front cover article.
Anion exchange membrane fuel cells (AEMFC) are devices that use hydrogen and oxygen to make clean electricity through chemical reactions, specifically the hydrogen oxidation reaction and the oxygen reduction reaction (ORR). AEMFCs, with their ability to operate in alkaline conditions, provide a suitable environment for earth-based catalysts, offering a cheaper alternative to other efficient catalyst materials, such as platinum.
Recent studies have shown that ZrN exhibits efficient performances—even outperforming platinum—when used for the ORR in alkaline media. ZrN, while not an Earth-abundant material, is still more cost-effective than alternatives. But what lay behind its impressive performance has remained a mystery to scientists.
“To implement our new theoretical framework for ZrN, we decided to employ surface state analysis, electric field effect simulations, and pH-dependent microkinetic modeling,” explains Hao Li, associate professor at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) and corresponding author of the paper.
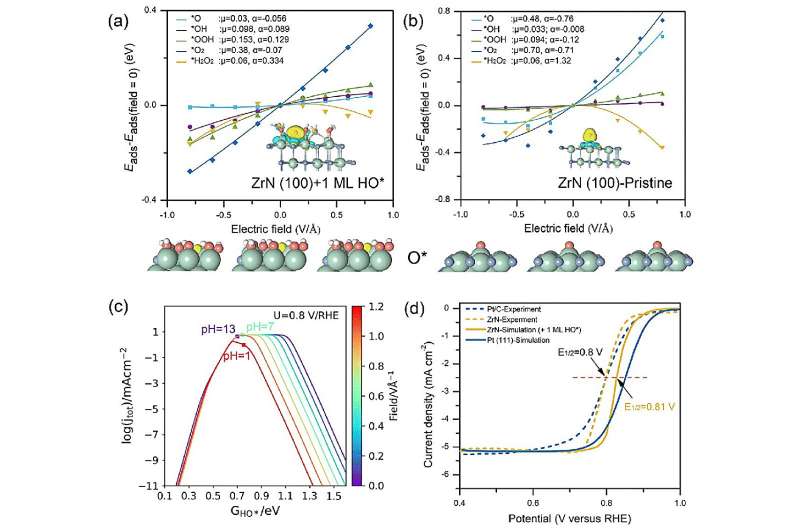
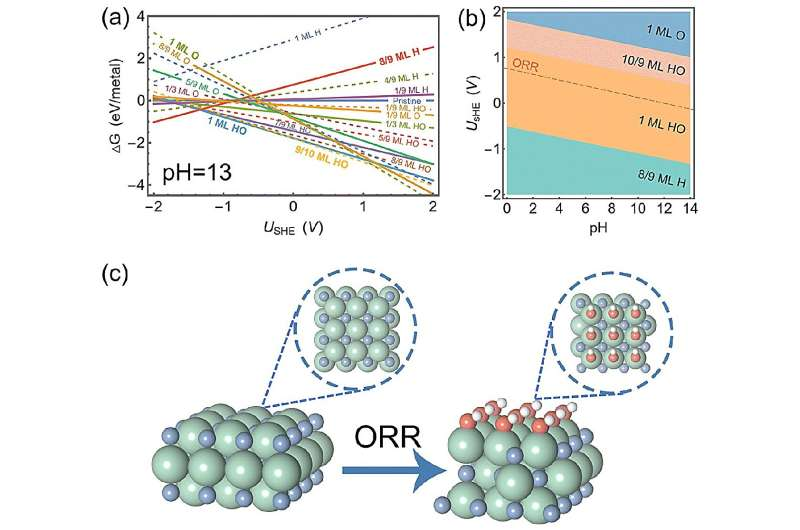
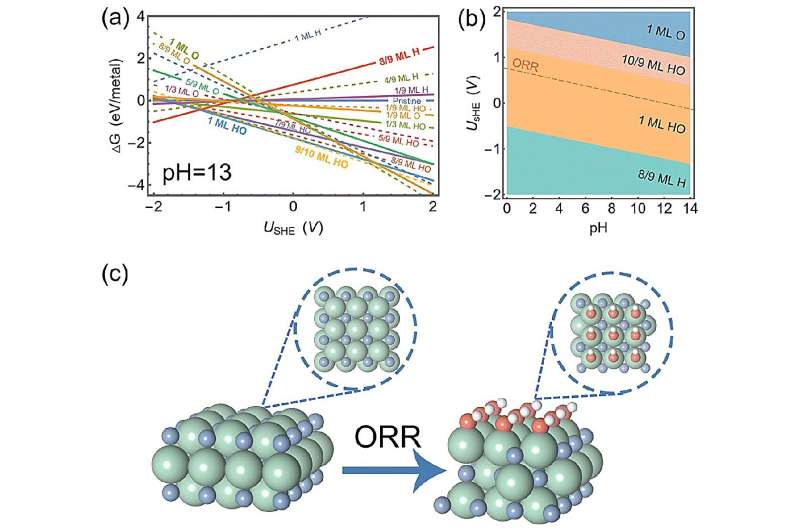
Surface analysis revealed that ZrN has a very thin layer of HO when it is undergoing ORR. This thin layer helps molecules stick to it in a way that is beneficial for the ORR. Moreover, the electric field effect simulations demonstrate that atomic oxygen sticking to this thin-covered surface undergo minimal changes, thereby sticking moderately.
After performing computer simulations, the researchers found that ZrN reaches the sweet spot of ORR in alkaline conditions.
“Our tested theory works well not just for ZrN but also for other materials like Fe3N, TiN, and HfN, which are similar to ZrN, meaning our idea explains how these materials can be utilized for clean energy too,” adds Hao. “Our framework will help rationalize and design transition metal nitrides for alkaline ORR.”
In the future, Hao and his team plan to extend this framework to study other industrially significant reactions, such as the oxygen evolution reaction.
More information: Heng Liu et al, Origin of the superior oxygen reduction activity of zirconium nitride in alkaline media, Chemical Science (2023). DOI: 10.1039/D3SC01827J
Journal information: Chemical Science
Provided by Tohoku University