by Liu Jia, Chinese Academy of Sciences
A research group led by Prof. Wan Yinhua from the Institute of Process Engineering of the Chinese Academy of Sciences has developed a catalyst with dual photocatalytic and biomimetic catalytic activity for the production of hydrogen peroxide (H2O2).
The strategy involves loading gold nanoparticles (AuNPs) onto graphite carbon nitride (GCN) nanosheets using polyethyleneimine (PEI) as a “bridge.” The study was published in the Chemical Engineering Journal.
H2O2, recognized as an environmentally friendly oxidant, is extensively used in various fields such as medical treatment, environmental restoration, fine chemicals, and electronics industries. However, the conventional method of H2O2 production relies on the anthraquinone process, which has several drawbacks including high energy consumption, the use of organic solvents, and safety hazards. Therefore, there is a pressing need to develop a sustainable and eco-friendly manufacturing process for H2O2.
Solar-driven photocatalysis is a promising alternative strategy for H2O2 production, and GCN is a popular choice in the field of photocatalysis due to its straightforward synthesis, cost-effectiveness, stable physical and chemical properties, and broad light absorption spectrum. However, GCN nanosheets alone have limited performance in photocatalytic H2O2 production in pure water due to the high energy barrier of water dissociation and low separation efficiency of charge carriers.
Hole sacrificial agents (acting as electron donors) such as ethanol, isopropanol, and benzyl alcohol are commonly used to improve the selectivity of oxygen reduction. However, the addition of organic agents has adverse environmental impacts, which is not conducive to the sustainability of H2O2 production. Therefore, it is crucial to engineer a GCN material with enhanced electron-hole pair separation efficiency to facilitate water oxidation and oxygen reduction.
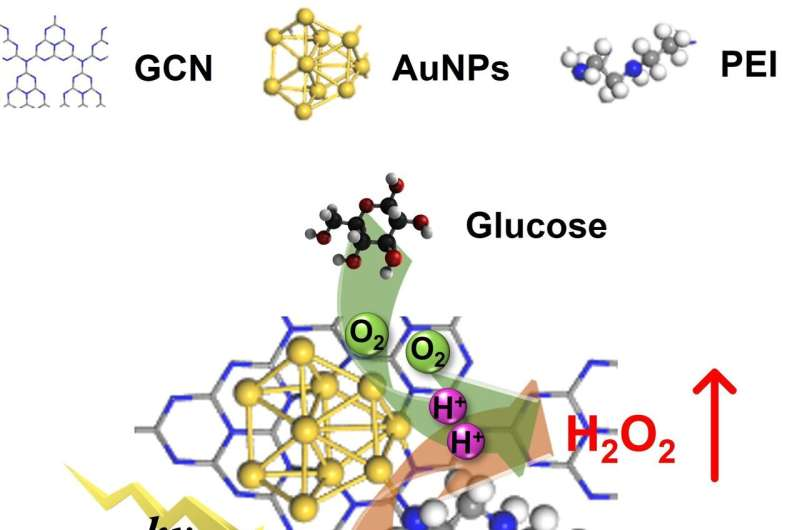
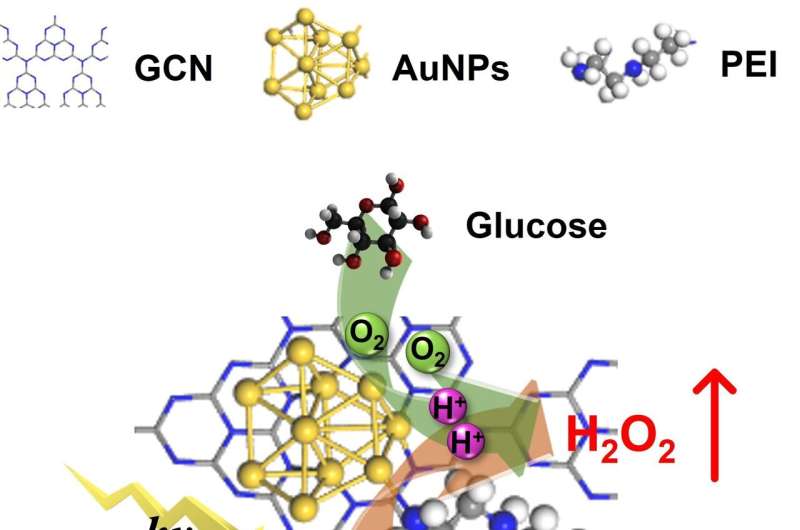
“Inspired by photo-enzyme-coupled catalytic system in chloroplasts, we developed a composite catalyst by loading AuNPs (enzyme mimics) on GCN (photocatalyst) nanosheets using PEI as the ‘bridge’ (PEI-GCN/Au),” Prof. Wan said.
The introduction of PEI and AuNPs helps adjust the electronic structure of GCN, facilitating the rapid separation of photogenerated carriers. The surface plasmon resonance of AuNPs, when excited by incident light, promotes the activation of glucose molecules, elevating their reactivity with O2 and improving the glucose oxidase-mimicking catalytic production of H2O2. The grafting of PEI and the addition of glucose enhance the O2 adsorption on the catalyst surface.
The PEI-GCN/Au composite demonstrates exceptional H2O2 production efficiency (270 μmol g-1 h-1) under visible light irradiation, using only glucose, H2O and O2 as reactants. As a result, both the biomimetic catalytic and photocatalytic reduction of O2 to H2O2 are enhanced, achieving a significant synergistic enhancement effect of 175%.
“This work establishes a paradigm of coupling biomimetic catalysis and photocatalysis for co-production of chemicals. It not only provides insights into the development of materials for efficient H2O2 production, but also introduces an innovative concept for integrating biocatalysis and photocatalysis,” said Prof. Luo Jianquan, the corresponding author of this study.
More information: Huiru Zhang et al, Biomimetic-photo-coupled catalysis for boosting H2O2 production, Chemical Engineering Journal (2024). DOI: 10.1016/j.cej.2024.149183
Provided by Chinese Academy of Sciences