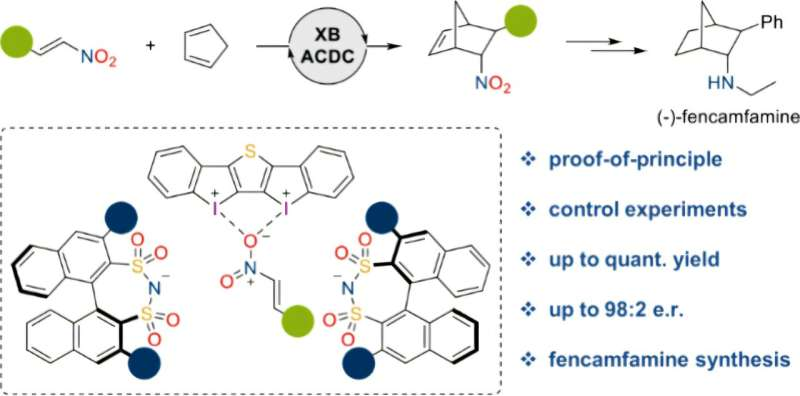
A research team based in Bochum and Mülheim is using a new type of salt to specifically produce one of two possible mirror-image molecules.
In scenarios where two mirror-image molecules are possible, special catalysts are required to produce only the desired one. A research team from the Ruhr Explores Solvation RESOLV Cluster of Excellence at Ruhr University Bochum, Germany, and the Max-Planck-Institut für Kohlenforschung in Mülheim, Germany, is using salts that control the desired reaction via halogen bonds.
Going forward, the salts can be adapted to different reactions as a modular system. The team headed by Professor Stefan Huber and Professor Benjamin List published their findings in the Journal of the American Chemical Society on March, 3, 2025.
A molecule and its twin
There are certain molecules that exist twice, so to speak: as themselves and as their mirror image. “These so-called chiral molecules demonstrate a handedness of sorts,” explains Huber. Despite sharing many similarities, they have very different properties, for example, in terms of their biological effectiveness. When used as a component of medicines, for example, the original molecule can have the desired effect, while its mirror image can cause adverse effects. This is why the aim is usually to produce only one variant of the molecule.
To this end, the team from Bochum and Mülheim adopted a novel approach. Their aim was to produce a molecule with interesting properties for medical application. The researchers used salts as catalysts in which both components (cation and anion) play an important role: The cation sets the reaction in motion via halogen bonds.
“Halogen bonds are weak bonds formed between the cation and the substrate,” explains Dominik Reinhard, Ph.D. student from Bochum. For its part, the anion, produced by the Mülheim researchers, ensures the correct handedness of the resultant molecule.
“The salt is introduced to the relevant substrates in a compatible solvent. The concerted action of the salt components then ensures the desired reaction, during which only the molecule with the desired handedness is formed,” explains Reinhard.
“What makes this approach so attractive is that, by using salts, we’ve created a modular system,” points out Huber. By combining a wide range of different cations and anions, the researchers can thus create different salts and, consequently, catalyze different reactions going forward.
More information: Dominik L. Reinhard et al, Asymmetric Counteranion-Directed Halogen Bonding Catalysis, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.4c18378
Journal information: Journal of the American Chemical Society
Provided by Ruhr-Universitaet-Bochum
