The face masks worn and discarded during the COVID-19 pandemic have an uncertain fate. Their physical damage to the environment and potential to trap organisms in ecosystems are significant concerns, but these are not the only issues. New research shows that the surrounding environment can change the chemical nature of the mask materials just as those materials can change the surrounding environment.
Disposable face masks, composed of polypropylene, can degrade into micro- and nanoplastics under sunlight, producing reactive oxygen species, highly potent oxidizing agents that can oxidize other environmental components and trigger unexpected reactions.
Recent research conducted by engineers at Washington University in St. Louis, led by Young-Shin Jun, a professor of energy, environmental and chemical engineering at the McKelvey School of Engineering, highlights the multipronged pollution problem posed by discarded face masks.
Ping-I (Dennis) Chou and Zhenwei Gao, both Ph.D. graduates of the McKelvey School of Engineering, are co-first authors of the work published in the Journal of Hazardous Materials. Their research started with a simple question: What happens to all those littered masks?
The study provides new insights about the significant chemical changes that occur when face masks are exposed to sunlight, water and trace metal ions.
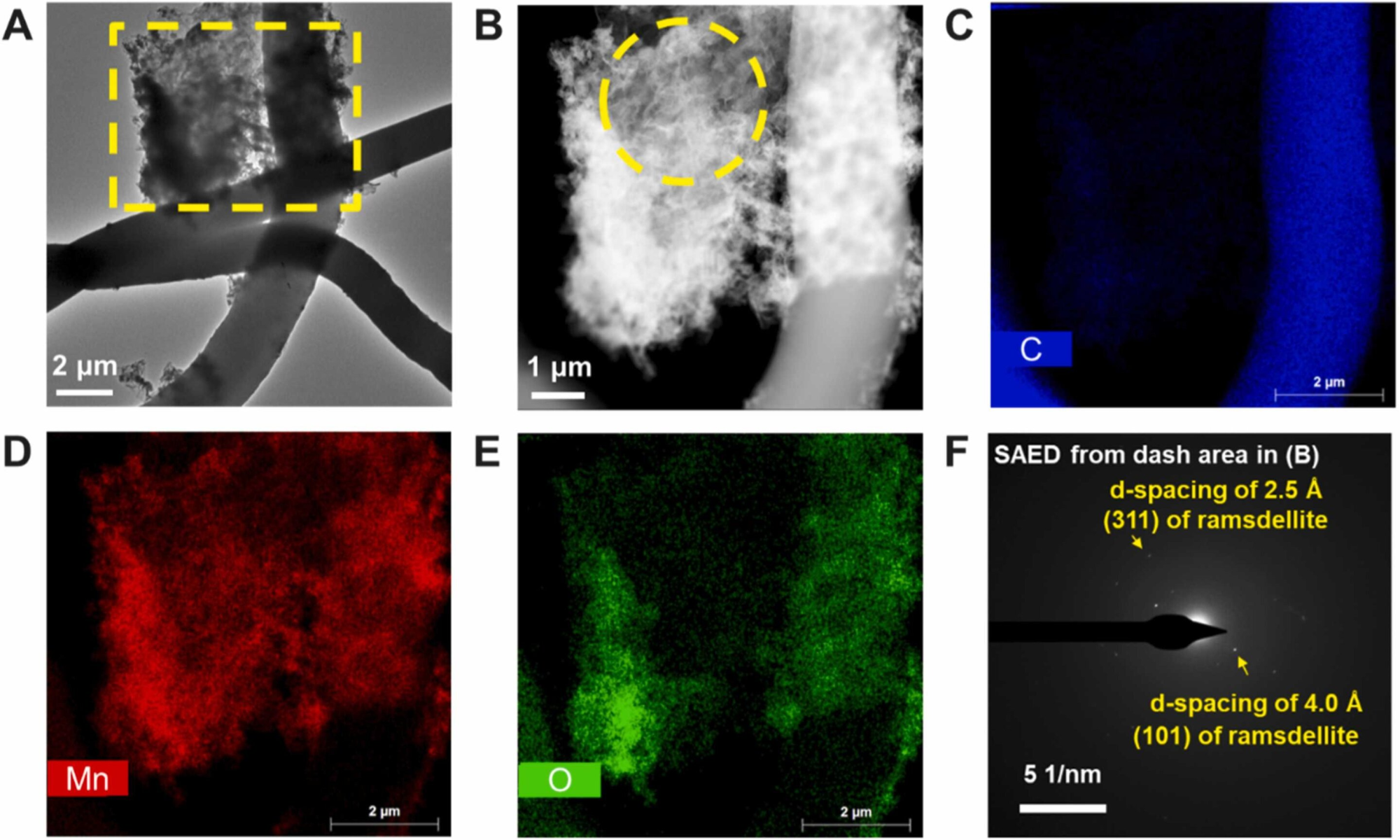
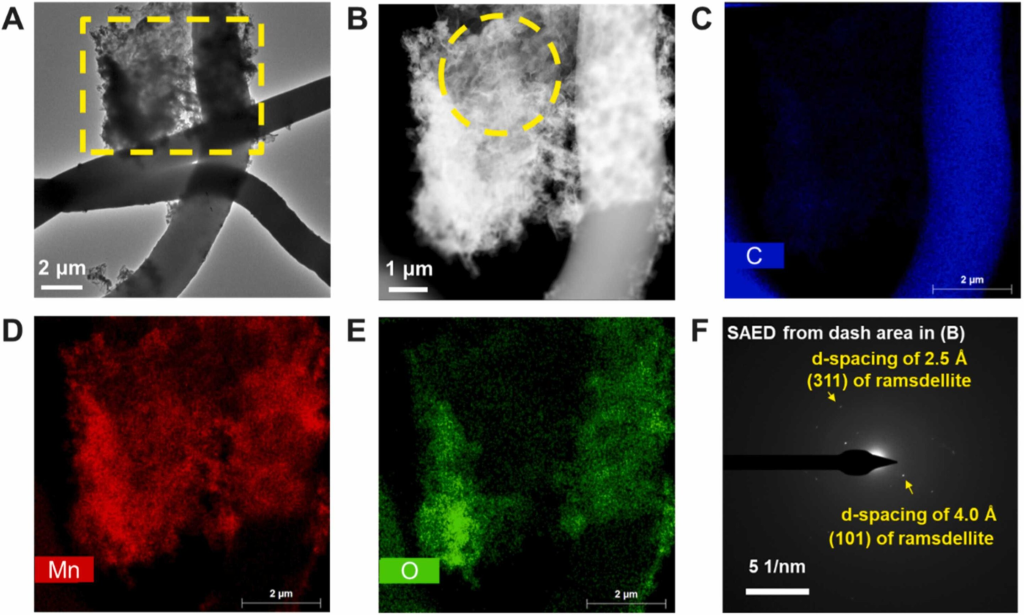
Masks degrade into nanoplastics and produce reactive oxygen species, Jun said. These newly formed, highly reactive oxidizing agents interact with metal ions, causing fast formation (within a few hours) of manganese oxide on the plastic particles, Jun explained.
She added that this study evaluated manganese ions because of their prevalence in the environment and applicability to other highly sensitive trace elements.
“These chemical reactions can change the reactivity and transport of these mask materials,” Jun said, “and thus how the materials will distribute will also change—something that has been generally overlooked.”
The environmental impact of face masks is concerning given the 2020 estimate that 1.56 million face masks entered the ocean. Manganese and iron drive various biogeochemical reactions and affect the surface chemistry of those materials. The interaction between these “redox elements” and plastic materials can influence the fate of both the plastics and trace metal species.
“They could alter the fate of trace metals, and simultaneously, trace metals could alter the fate of microplastics,” Jun added.
Also, among their findings: exposure to sunlight is required for that ultrafast manganese oxide formation.
In the future, Jun and her research team will explore how organic components in aquatic environments affect the transformation and transport of pollutants from face masks. She is also interested in how biofilms of microbes interact with metal-coated nanoplastics and the role of different polymer structures in plastic waste in influencing the fate and transport of those reactive metal ions.