Hydrogen peroxide, a key chemical used in the semiconductor production process, is one of the top 100 industrial chemicals and an important raw material widely used in disinfection, oxidation, and pulp manufacturing. The global hydrogen peroxide market is expected to exceed 7 trillion won (KRW) in 2024 (approximately $5.5 billion USD).
However, it is predicted that stable supply of hydrogen peroxide will be difficult to achieve due to the recent worldwide COVID quarantine measures and rapid increase in demand for semiconductor production. Moreover, the current production method for hydrogen peroxide is a thermochemical process (anthraquinone process), which uses palladium, an expensive rare metal, as a catalyst at high temperature and pressure. This process not only consumes a lot of energy, but also causes various environmental problems such as the risk of explosion and emission of greenhouse gases.
Although many efforts have been made to produce hydrogen peroxide with low energy consumption and low carbon emission, it is a challenge to overcome the threshold of commercialization due to extremely low productivity and efficiency. Hence, there is an urgent need to develop eco-friendly technologies that can solve the problems of existing thermochemical processes.
The Korea Institute of Science and Technology (KIST) announced last November that Dr. Jeehye Byun’s research team at the Center for Water Cycle Research and Dr. Dong Ki Lee’s research team at the Clean Energy Research Center developed a new technology that uses sunlight to produce hydrogen peroxide at an unprecedented high concentration, replacing the need for high-temperature and high-pressure energy. This technology is an example of replacing a thermochemical process with a photocatalytic process to produce key chemical raw materials without carbon emissions.
The KIST research team designed the photocatalytic reaction solution as an organic solution based on the fact that anthraquinone organic molecules undergo repeated oxidation and reduction reactions in the existing thermochemical process to produce hydrogen peroxide. As a result, they discovered that the oxygen reduction ability of the photocatalyst was improved in the organic reaction solution, and hydrogen peroxide production was greatly increased. In addition, the research team identified for the first time that the organic reaction solution itself absorbs light and produces hydrogen peroxide through a photochemical reaction.
The research team achieved the result of producing hydrogen peroxide at a concentration of 53,000 ppm (i.e., 5.3%) per unit time and per gram of photocatalyst by using sunlight when controlling the photocatalyst and reaction solution. This is an achievement that exceeds the hydrogen peroxide production industry standard of at least 10,000 ppm, or 1%, by more than five times.
Therefore, this is a breakthrough performance figure considering that the existing photocatalyst technology only produces hydrogen peroxide at the level of tens to hundreds of ppm. This technology achieved a solar-to-chemical conversion efficiency of 1.1% through the synergistic effect of two photoreactions, i.e., photocatalyst and photochemistry, breaking the world’s highest efficiency as well as the previous photocatalyst’s highest efficiency of 0.61%.
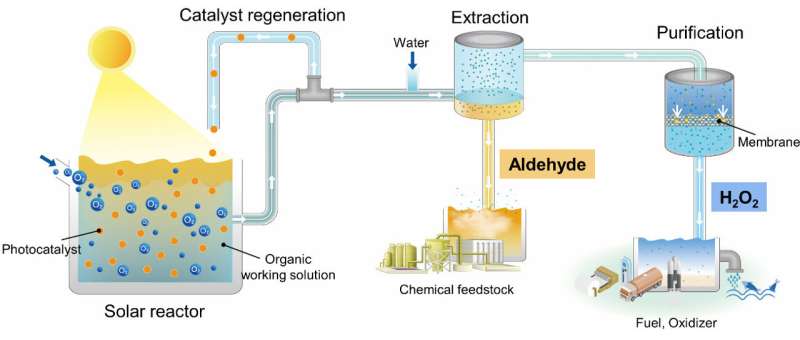
Dr. Byun and Dr. Lee of KIST said that “This study proves that low-carbon, eco-friendly technology using sunlight can also produce core industrial fuels with high concentration and purity.” They also stated, “We verified the completeness of the technology by linking the process of refining the produced hydrogen peroxide to a liter scale, and we will strive to commercialize the technology through large-scale demonstration in the future.”
The research is published in the journal Energy & Environmental Science.
More information: Byeong Cheul Moon et al, Solar-driven H2O2 production via cooperative auto- and photocatalytic oxidation in fine-tuned reaction media, Energy & Environmental Science (2022). DOI: 10.1039/D2EE02504C
Journal information: Energy & Environmental Science
Provided by National Research Council of Science & Technology