As one of the most efficient and environmentally friendly approaches to hydrogen production, water electrolysis consists of two half reactions: hydrogen evolution reaction and oxygen evolution reaction (OER).
In order to accelerate the OER, Prof. Chen Liang’s team at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences (CAS) has conducted series research on OER electrocatalysis by combining experiment and theoretical calculation.
Compared with the alkaline water electrolysis, proton exchange membrane (PEM)-based water electrolysis has distinct advantages such as compact design, fast response, high voltage efficiency and high gas purity. However, the limited efficiency, low stability and high cost of acidic OER electrocatalysts still hinder the development of PEM-based water electrolysis.
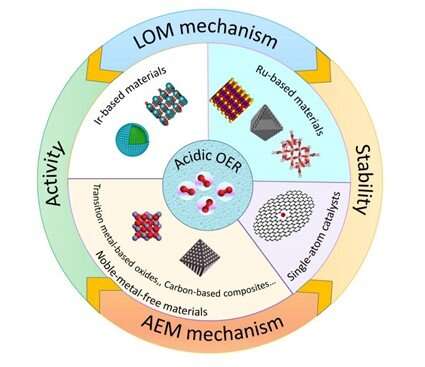
Based on previous studies on electrocatalysts for OER, researchers at NIMTE have comprehensively summarized, classified and discussed the recently reported acidic OER electrocatalysts in terms of elements. The study was published in Advanced Materials.
Great emphasis was placed on the OER mechanisms, which fall into two categories: adsorbate evolution mechanism and lattice oxygen oxidation mechanism, according to the origin of oxygen atoms.
The relationship between activity and stability of acidic OER electrocatalysts was discussed in detail. In addition, the researchers proposed a stability test protocol to evaluate the intrinsic activity degradation.
The current development challenges and unresolved issues of acidic OER electrocatalysts were discussed, such as the use of carbon-based materials. The proposals for the synthesis of high-performance acidic OER electrocatalysts for PEM-based water electrolysis were also presented.
More information: Yichao Lin et al, Electrocatalysts for Oxygen Evolution Reaction in Acidic Media, Advanced Materials (2022). DOI: 10.1002/adma.202210565
Journal information: Advanced Materials
Provided by Chinese Academy of Sciences
