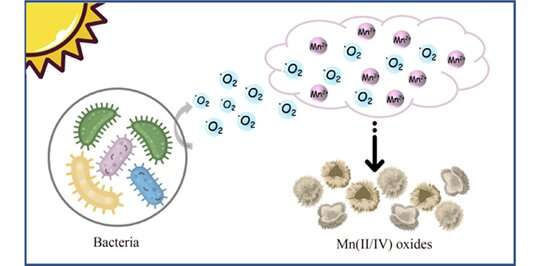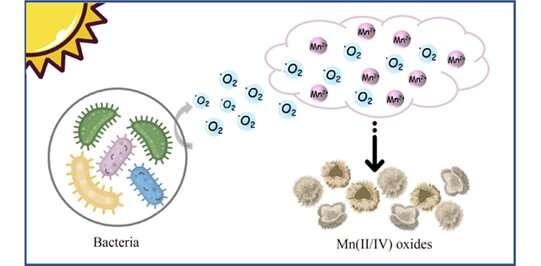
Manganese oxides are natural reactive minerals and widely spread in aquatic and terrestrial environments, affecting the fate of metals (such as As3+ and Cd2+) and organic pollutants (such as phenols and diclofenac) through adsorption and oxidation in sewage treatment. Usually, the manganese (III/IV) oxides in the environment are thought to be formed by the oxidation of dissolved Mn(II) through abiotic or biotic processes.
Oxidation of aqueous Mn(II) by dissolved oxygen is thermodynamically favored, but the kinetic is slow due to the high energy barrier of the reaction from dissolved Mn(II) to Mn(III/IV) oxides. The presence of microorganisms accelerates the oxidation rate, which is 4–5 orders of magnitude faster than the rate of abiotic chemical oxidation, therefore is considered as the initial source of manganese oxides in the environment.
Bacteria capable of catalyzing the oxidation of dissolved Mn(II) ions to undissolved Mn(III/IV) oxides are usually called manganese-oxidizing bacteria. The bacterial oxidation of Mn(II) ions are divided into direct and indirect ways, and the process catalyzed by enzymes on the surface of microorganisms is called direct oxidation. For indirect pathways, some bacteria can change their surrounding environmental conditions for Mn(II) oxidation (e.g., the pH and Eh).
Roseobacter clade has been demonstrated to oxidize Mn(II) by producing extracellular reactive oxygen species in recent studies. Do other bacteria clades have similar Mn(II) oxidation processes with Roseobacter? Is the Mn(II) oxidation closely relative to the physiological process of bacteria?
To answer these questions, Prof. Feng Zhao from Chinese Academy of Sciences and his team members explored the microbial manganese oxidation process under visible light by using coastal surface seawater microorganisms. The relationship between the transformation of soluble Mn(II) into insoluble Mn(III/IV) oxides by microorganisms and physiological role was analyzed. This study is published in Frontiers of Environmental Science & Engineering in 2023.
In this study, the research team found visible light greatly promotes the oxidation rate of Mn(II), and the average rate reaches 64 μmol/(L·d). The generated manganese oxides were then conducive to Mn(II) oxidation, thus the rapid manganese oxidation was the result of the combined action of biotic and abiotic, and biological function accounts for 88 % ± 4 %.
Extracellular superoxide produced by microorganisms induced by visible light is the decisive factor for the rapid manganese oxidation in our study. But the production of these superoxides does not require the presence of Mn(II) ions, the Mn(II) oxidation process was more like an unintentional side reaction, which did not affect the growth of microorganisms.
More than 70 % of heterotrophic microorganisms in nature are capable of producing superoxide, based on the oxidizing properties of free radicals, all these bacteria can participate in the geochemical cycle of manganese. What’s more, the superoxide oxidation pathway might be a significant natural source of manganese oxide.
This study revealed an essential pathway for bacterial manganese oxidation. Heterotrophic bacteria produce superoxide under visible light irradiation and oxidize Mn(II) ions in the surrounding environment, which is the main source of manganese oxides. The biogenerated Mn(III/IV) oxides can also oxidize Mn(II) ions indirectly through abiotic reactions under light illumination.
Many bacteria in the environment that produce superoxide actively or passively may also oxidize Mn(II) in this way, suggesting that the manganese oxidation pathway through superoxide is a common behavior in the environment. In light of the oxidation properties and semiconductor properties of manganese oxides, this research will provide new ideas for the treatment of environmental pollution.
More information: Fan Yang et al, Visible light induces bacteria to produce superoxide for manganese oxidation, Frontiers of Environmental Science & Engineering (2022). DOI: 10.1007/s11783-023-1619-y
Provided by Higher Education Press