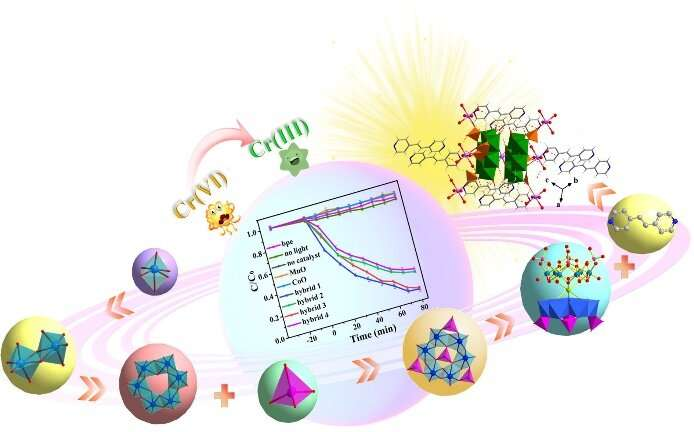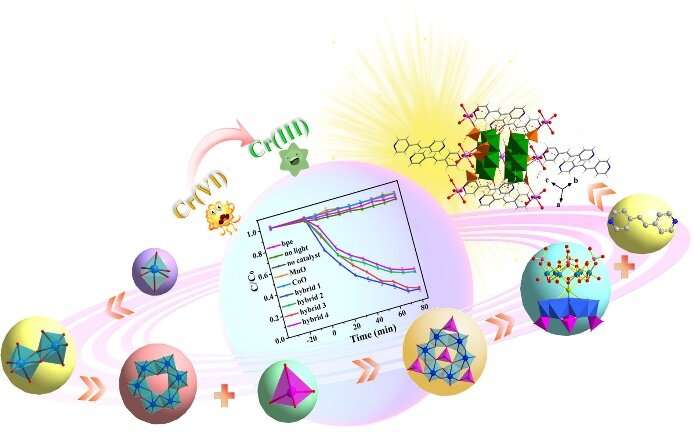
Toxic heavy metals found in wastewater have health and safety ramifications for communities affected by pollution. Hexavalent chromium is a dangerous, cancer-causing byproduct of industrial processes that is known to cause birth defects, severe diarrhea, and is linked to kidney, bladder, and liver cancers. Famously, it was the center of the lawsuit dramatized in the film “Erin Brockovich.”
Researchers are trying to find effective ways to remove hexavalent chromium from wastewater and a recently published paper shows how photocatalytic technology may be a solution. Photocatalysis is when light and a catalyst are used to speed up chemical reactions.
The paper was published in Polyoxometalates.
“Rapid industrialization causes an increased release of wastewater containing heavy metal ions. Hexavalent chromium, which has high carcinogenicity and teratogenicity, is widely found in wastewater and can easily enter food chains,” said Yuan-Yuan Ma, a researcher at Hebei Normal University in Shijiazhuang, China. Photocatalysis technology is an appealing solution for removing heavy metals from wastewater because it is sustainable, cost-effective, and environmentally friendly.
“This green approach for the removal of heavy metal ions uses sustainable light energy via hourglass-type phosphomolybdate-based crystalline photocatalysts and develops a strategy for the regulation of photocatalytic performance by adjusting the central metal ions in hourglass-type phosphomolybdate clusters,” said Ma. Researchers chose this particular type of photocatalyst because of its molecular properties and well-defined hourglass-type structure, which give it a wide light absorption ability and the band structure necessary to reduce the levels of hexavalent chromium.
Researchers tested four “hybrid” photocatalysts and compared them to six other photocatalysts. The hybrids had slightly different compositions, but all had the same hourglass-type structure that could be maintained up to 200 degrees Celsius. They had wide visible-light absorption and similar energy band structures. Researchers labeled these as Hybrid 1, 2, 3, and 4. After 80 minutes of exposure to a 10W LED light, hybrid 1 and 3 had around a 90% reduction in hexavalent chromium, while 2 and 4 had around a 74% and 71% reduction in hexavalent chromium respectively.
The hybrids generally performed better than any of the tested photocatalysts. Hybrids 1 and 3, which performed best, both were Mn{P4MO6}2-based hybrids. Hybrids 2 and 4 were Co{P4MO6}2-based. Researchers suspect that the better performance was due to structural differences that gave hybrids 1 and 3 a narrower band gap. “The semiconductor photocatalysts in photocatalytic processes can absorb photons matched with their band gap energy, leading to the conversion of toxic hexavalent chromium to less toxic chromium,” said Ma.
Looking ahead, researchers will focus on improving the design of the photocatalysts, while also planning for how to best bring this technology to a real-world wastewater setting. “Designing effective photocatalysts is the first step to solve heavy metal pollution in water,” said Ma. “We will design more efficient photocatalysts and apply the developed photocatalysts to actual industrial wastewater. We will also expand the treatment range of polluted water sources and strive to realize the practicality of the photocatalyst materials used.”
More information: Xiao-Yu Yin et al, Photoactive hourglass-type M{P 4Mo 6} 2 networks for efficient removal of hexavalent chromium, Polyoxometalates (2023). DOI: 10.26599/POM.2023.9140027
Provided by Tsinghua University Press