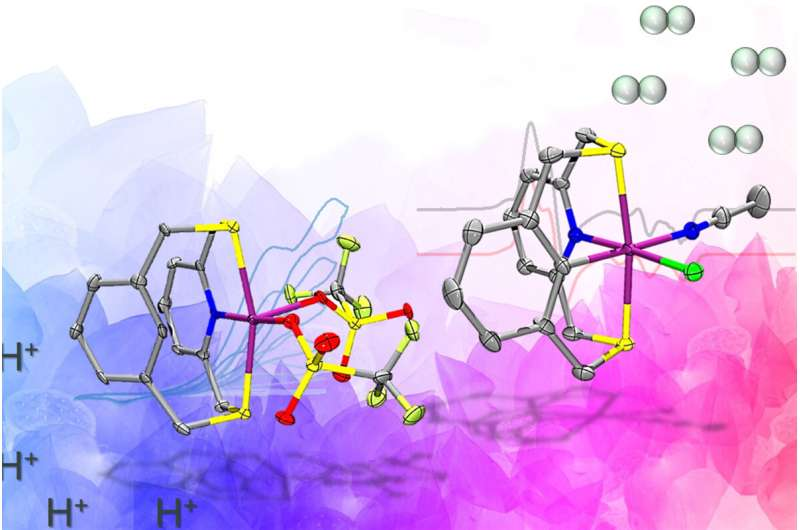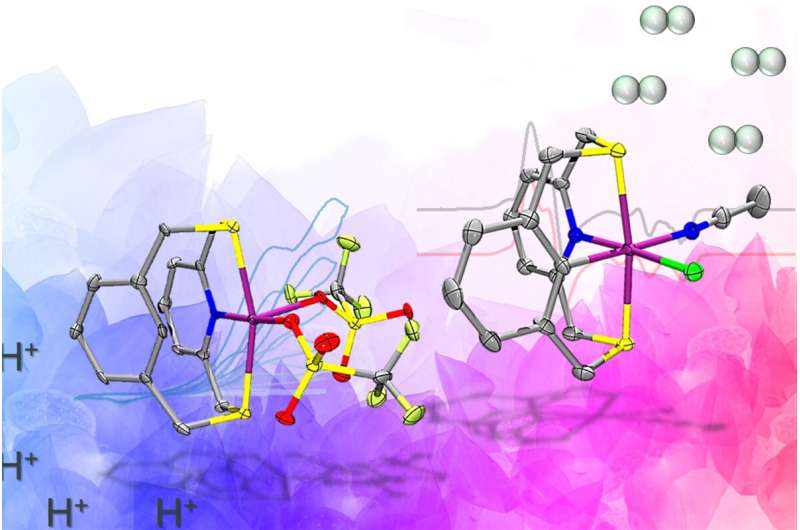
An ancient biological enzyme known as nickel-iron hydrogenase may play a key role in producing hydrogen for a renewables-based energy economy, researchers have found. Careful study of the enzyme has led chemists from the University of Illinois Urbana-Champaign to design a synthetic molecule that mimics the hydrogen gas-producing chemical reaction performed by the enzyme.
The researchers reported their findings in the journal Nature Communications.
Currently, industrial hydrogen is usually produced by separating hydrogen gas molecules from oxygen atoms in water using a process called electrolysis. To boost this chemical reaction in the industrial setting, platinum metal is used as a catalyst in the cathodes that direct the reaction. However, many studies have shown that the expense and rarity of platinum make it unattractive as the world pushes toward more environmentally sound energy sources.
On the other hand, nature’s nickel-iron hydrogenase enzyme produces hydrogen using earth-abundant metals in its core, said chemistry professor Liviu Mirica, who led the study with graduate student Sagnik Chakrabarti.
“The nickel at the core of the natural enzyme produces hydrogen by reducing protons in water,” Chakrabarti said. “During the catalytic process, the nickel center goes through paramagnetic intermediates, meaning that the intermediates have an unpaired electron—which makes them extremely short-lived.”
Synthetic chemists have made nickel compounds that produce hydrogen for over a decade, Mirica said. While some of these compounds are very efficient at producing hydrogen, the vast majority of them operate via intermediates that are not paramagnetic.
“Researchers are trying to mimic exactly what nature does because it is efficient, and maximizing efficiency is a key challenge to overcome when engineering energy sources,” Mirica said. “Being able to reproduce the paramagnetic intermediate steps that occur in the natural enzyme is what our group is trying to achieve—to increase efficiency and mimic nature.”
To achieve this, the team designed an organic molecule called a ligand that contains electron-donating atoms like nitrogen and sulfur, and can hold the nickel in place and support the two relevant paramagnetic states that produce hydrogen. The key design element that sets this molecule apart from other catalysts is the presence of a carbon-hydrogen bond near the nickel center that is broken and formed again during catalysis. This was crucial in stabilizing the aforementioned paramagnetic states.
“One of the key takeaways from our work is that by using the specially designed ligand in the manner we did, we have successfully united ideas from two fields of inorganic chemistry—bioinorganic and organometallic chemistry—to make nickel complexes that behave similarly to the active site of one of nature’s most beautiful and complicated enzymes,” Chakrabarti said.
In the recent past, several unusual enzymes have been found that feature metal-carbon bonds in their active sites, the researchers said. Such design principles in synthetic complexes could lead to further insights into how nature performs chemistry with small molecules like hydrogen.
Former Illinois researchers Soumalya Sinha, Giang N. Tran and Hanah Na contributed to this study.
More information: Sagnik Chakrabarti et al, Characterization of paramagnetic states in an organometallic nickel hydrogen evolution electrocatalyst, Nature Communications (2023). DOI: 10.1038/s41467-023-36609-7
Journal information: Nature Communications
Provided by University of Illinois at Urbana-Champaign