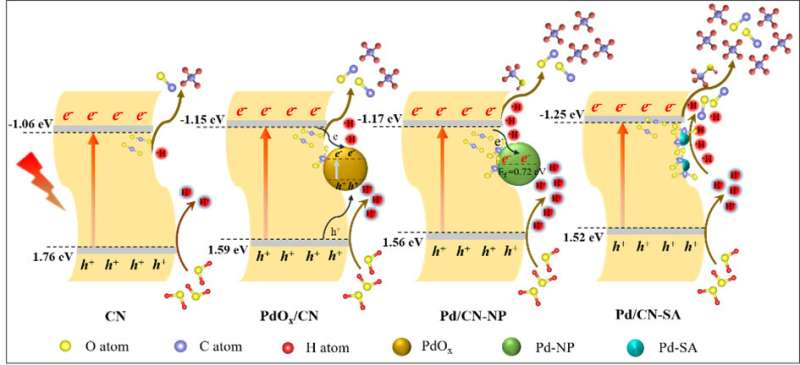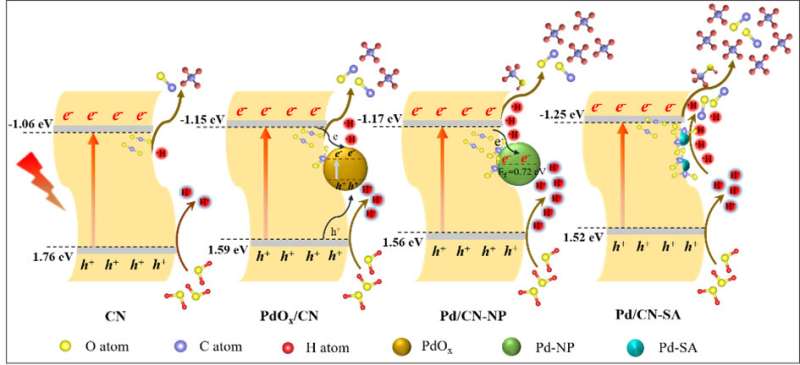
Using solar energy and photocatalysts to convert CO2 into high value-added chemicals can simultaneously alleviate the greenhouse effect and energy crisis. Single atom cocatalysts decoration has been demonstrated to be an effective strategy to improve the CO2 photocatalytic reduction efficiency.
Unfortunately, when unraveling the mechanism behind performance promotion, most studies mainly focus on clarifying the superior physicochemical and photoelectrical properties of SACs in comparison with the substrate. The critical role of the sing-atomic state distinguished from those oxide and elemental states was often neglected and remains a mystery.
Recently, a research team led by Prof. Zhongbiao Wu and Haiqiang Wang from Zhejiang University, China, comprehensively investigated the effect of Pd chemical states on CO2 photocatalytic reduction of g-C3N4 (CN) under visible light irradiation, especially the critical role of Pd-SA in boosting CH4 production. The results were published in the Chinese Journal of Catalysis.
Performance tests showed Pd species decoration improved the CH4 production of CN, with Pd/CN-SA exhibiting the optimum yields (2.25 μmol g-1), markedly higher than that of PdOx/CN (1.08 μmol g-1) and Pd/CN-NP (0.44 μmol g-1). After comprehensive mechanism analysis with various characterization techniques, in-situ FTIR spectra and DFT calculations, it was found that the conducive activation of CO2, negative conduction band potentials, and excellent •H utilization efficiency, collaboratively contributed to the superior CO2 reduction performance of Pd/CN-SA, especially in the remarkably boosted CH4 production.
In addition, despite the larger electron density of Pd/CN-NP and PdOx/CN, the moderate reduction ability of their photogenerated electrons restricted the further reduction of adsorbed CO2 species and CO intermediate, limiting the enhancement of CO2 reduction activity. Furthermore, the CH4 evolutions of Pd/CN-NP and PdOx/CN were also limited by the poor •H supply and inferior •H utilization efficiency, respectively.
The new insights may advance the understanding of CO2 reduction process and inspire the design of efficient photocatalysts for CO2 photocatalytic conversion.
More information: Qian Li et al, Effect of palladium chemical states on CO2 photocatalytic reduction over g-C3N4: Distinct role of single-atomic state in boosting CH4 production, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(22)64199-8
Provided by Chinese Academy of Sciences