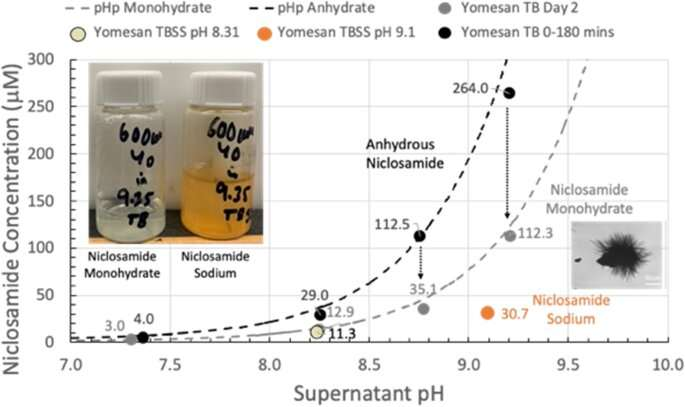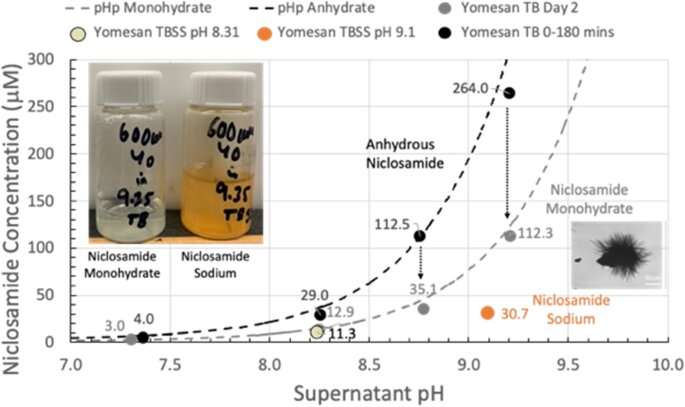
David Needham, professor of mechanical engineering and materials science at Duke University, has demonstrated that a metabolic inhibiting drug called niclosamide, traditionally used to treat gut parasites, can readily be extracted and dissolved from commercial tablets in quantities sufficient to create throat and nasal sprays.
Combined with previous research that shows niclosamide might be able to prevent or inhibit the growth cycle of common respiratory viruses—including COVID-19—the results may point toward an easier pathway through the testing, regulatory approval and manufacturing processes needed to bring a potential product to market.
The research was published online April 14 in the journal American Association of Pharmaceutical Scientists Open (AAPS Open).
“These results show a potentially more rapid route to FDA approval, bringing a new commercial opportunity that could make the nasal sprays more readily available worldwide,” Needham said. “This would not just potentially be for COVID-19, but also for all respiratory viruses including Influenza and Respiratory Syncytia Virus (RSV) and to increase our preparedness for the next pandemic that seems sure to be coming down the pipe.”
Since 1958, niclosamide has been used to treat gut parasite infections in humans as well as pets and farm animals. Delivered as oral tablets, the drug kills the parasites on contact by inhibiting their crucial metabolic pathway and shutting down their energy supply.
In recent years, however, researchers have been testing niclosamide’s potential to treat a much wider range of diseases, such as many types of cancer, metabolic diseases, rheumatoid arthritis and systemic sclerosis. Recent laboratory studies in cells have also shown the drug to be a potent antiviral medication, inhibiting a virus’s ability to cause disease by targeting the energy supply of the host cell that the virus co-opts for its self-replication.

Needham showed in 2022 that the drug could be dissolved into high enough concentrations to create a potential throat or nasal spray by a simple change in the solution’s pH. This was an important result, as researchers had previously believed the drug to be too insoluble to form such solutions.
Needham is now working with colleagues Zachary Kelleher in the lab of Christina Barkauskas, assistant professor of medicine in pulmonary medicine at Duke, to evaluate how niclosamide brings down the available energy in human nasal and bronchial cells. The team is also working to show that niclosamide is safe at concentrations above those where it has been found in the literature to prevent viral infection. They are now testing the effects of niclosamide in the Duke Regional Biocontainment Laboratory to see if it can prevent infection in more respiratory-relevant cells.
“Academic papers and companies actively involved in potential niclosamide products were automatically invoking much more complex formulations,” Needham said. “I showed that you could raise the solubility to what you would want for this spray-style application.”
Because the tablets are already FDA approved, the spray solution formulation seemed set for a straightforward approval and launch into a safety-efficacy clinical trial. However, the FDA viewed this throat/nasal spray as a new formulation that needed to be tested from square one, despite its concentrations being millions of times lower than the oral tablets that have been approved for more than 50 years.
By showing that enough niclosamide can be extracted from this already-approved formulation, Needham is hoping to expedite the testing and approval process.
“Having laid out the way to make the throat/nasal spray solutions by the simplest of techniques and showing that it can be readily scaled up to liters of volumes, my hope is that one or more companies will recognize not only the commercial opportunity, but also that this is the right thing to do to save lives and reduce suffering across the planet,” Needham said.
Moving forward, Needham is working to optimize these niclosamide-based solutions with an additional depot of dissolvable solid microparticles of niclosamide so that the throat and nasal tissue is continually supplied with safe concentrations of the drug. He’s also looking to engage public labs, companies, institutes and governments to make and test the formulations.
More information: David Needham, Extraction of niclosamide from commercial approved tablets into aqueous buffered solution creates potentially approvable oral and nasal sprays against COVID-19 and other respiratory infections, AAPS Open (2023). DOI: 10.1186/s41120-023-00072-x
Provided by Duke University