A screening technique commonly used in drug discovery can yield important details about the actions of molecular ‘glues’ in protein interactions.
Molecular glues are emerging as powerful therapeutic tools that can stick proteins together in the body. The interactions between proteins underpin all biological cell functions, including those of disease, and so interventions that can control protein-protein interactions have significant potential for disrupting the progress of various diseases.
While in many cases, drugs are required to interrupt the processes that connect proteins together, there are also occasions when the intervention is needed to restore an interaction, or to make it function correctly.
Researchers at the University of Birmingham, together with partners at the University of Leicester and the Eindhoven University of Technology, have devised a way of using mass spectrometry to analyze candidate glues for these processes and assess their relative strengths.
Dr. Aneika Leney, of the School of Biosciences at the University of Birmingham, explained, “Often when we are designing new drugs, it is to stop harmful protein interactions in the body, such as those that lead to tumor cell growth in cancers. Sometimes, however, the disease is caused by protein interactions falling apart and in these cases finding the right glue to hold them together could be extremely beneficial.”
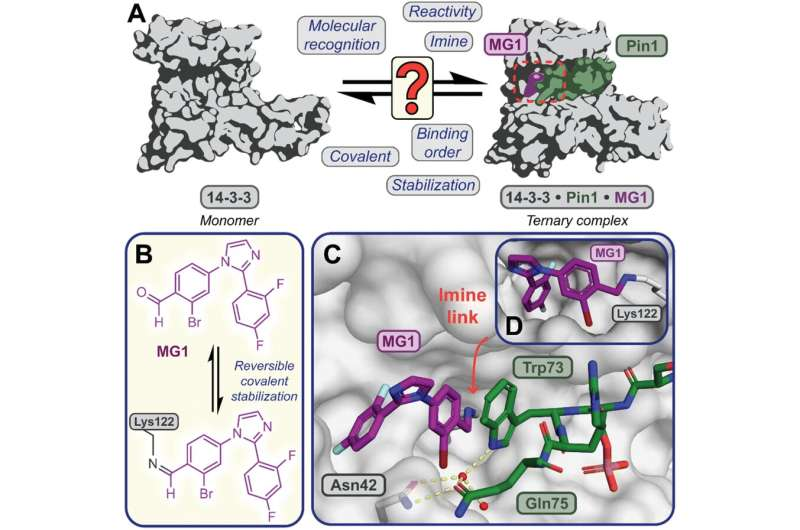
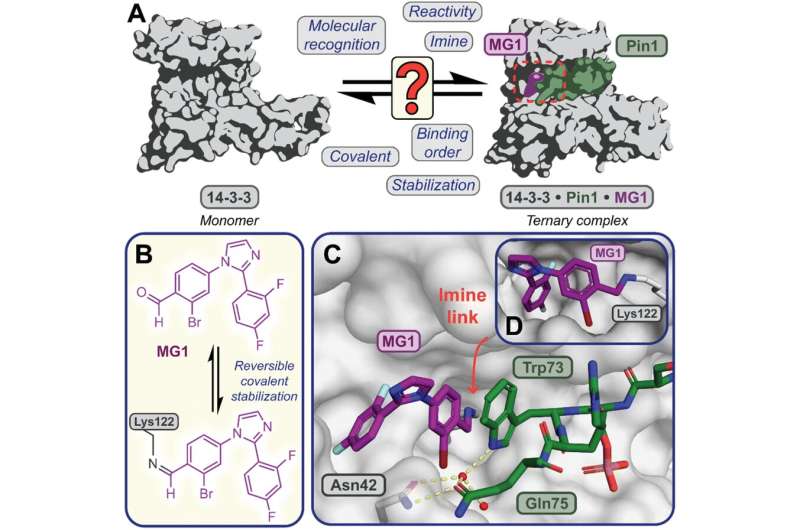
In a new study, published in Chemical Science, the research team focused on one particular molecular glue, called MG1. Using the mass spectrometry method, they were able to disentangle the different mechanisms through which the glue bound to the proteins and stabilized the protein interaction. The MS method also allowed the researchers to elucidate the relative time taken by the different processes involved.
Dr. Peter Cossar, from the Department of Biomedical Engineering at Eindhoven University of Technology further explained, “Understanding how molecular glues stick proteins together enables scientist to better design and build the next generation of molecular glue drugs. Mass Spectrometry provides a tool to do so, by providing high fidelity information on how these unique molecules behave in real time.”
The team expect that the research will provide a robust framework for testing a wide range of molecular glues, offering a significant advance in drug discovery understanding in this area.
More information: Carlo J. A. Verhoef et al, Tracking the mechanism of covalent molecular glue stabilization using native mass spectrometry, Chemical Science (2023). DOI: 10.1039/D3SC01732J
Journal information: Chemical Science
Provided by University of Birmingham