New research by the University of Liverpool’s Chemistry Department represents an important breakthrough in the field of polymer science.
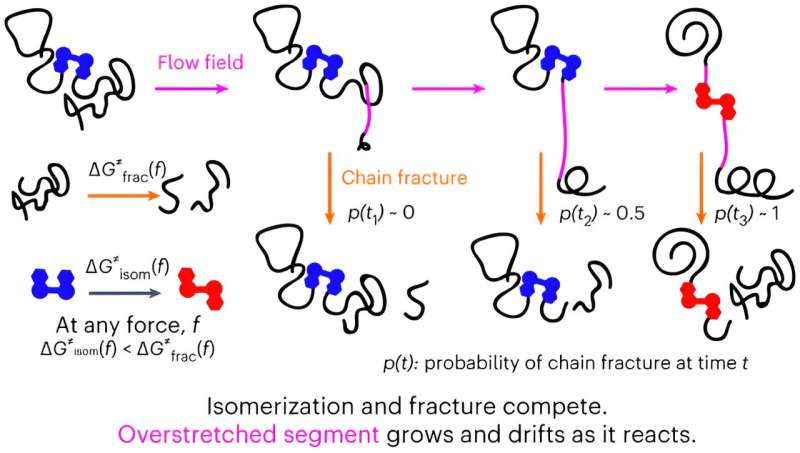
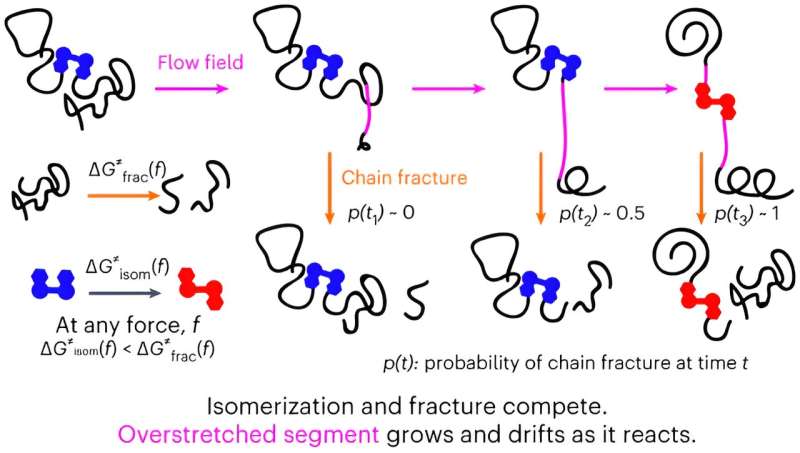
In their study, Liverpool researchers use mechanochemistry to characterize how a polymer chain in solution responds to a sudden acceleration of the solvent flow around it.
The paper, “Experimental quantitation of molecular conditions responsible for flow-induced polymer mechanochemistry,” is published in Nature Chemistry and is featured on the front cover.
This new approach answers a fundamental and technological question that has preoccupied polymer scientists for the past 50 years.
Since the 1980s, researchers have been trying to understand the unique response of dissolved polymer chains to suddenly accelerating solvent flows but had been constrained to highly simplified solvent flows that provided limited exploitable insights into the behavior of real-world systems.
The new discovery by Liverpool chemists Professor Roman Boulatov and Dr. Robert O’Neill has significant scientific implications for several areas of physical sciences as well as at a practical level for polymer-based rheological control used in many multi-million dollar industrial processes such as enhanced oil and gas recovery, long distance piping and photovoltaics manufacturing.
Professor Roman Boulatov said, “Our finding addresses a fundamental and technical question in polymer science and potentially upends our current understanding of chain behavior in cavitational solvent flows.”
Co-author of the paper, Dr. Robert O’Neill added, “Our proof-of-the-approach demonstration reveals that our understanding of how polymer chains respond to sudden accelerations of solvent flows in cavitating solutions was too simplistic to support systematic design of new polymer structures and compositions for efficient and economical rheological control in such scenarios or for gaining fundamental molecular insights into flow-induced mechanochemistry.
“Our paper has important implications for our ability to study non-equilibrium polymer chain dynamics at the molecular length scales, and thus our capacity to answer fundamental questions of how energy flows between molecules and within them, and how it transforms from kinetic to potential to free energies.”
The research team plans to focus on expanding the scope and capabilities of their new method and exploiting it to map molecular-level physics that would allow accurate predictions of flow behavior for an arbitrary combination of polymer, solvent and flow conditions.
More information: Robert T. O’Neill et al, Experimental quantitation of molecular conditions responsible for flow-induced polymer mechanochemistry, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01266-2
Journal information: Nature Chemistry
Provided by University of Liverpool