by David Nutt, Cornell University
Christopher Parzyck had done everything right. Parzyck, a postdoctoral researcher, had brought his nickelate samples—a newly discovered family of superconductors—to a synchrotron beamline for X-ray scattering experiments. He was measuring his samples, which he’d synthesized with a new method, in the hope of detecting the suspected presence of “charge ordering”—a phenomenon in which electrons self-organize into periodic patterns. The phenomenon has been linked to high-temperature superconductivity.
But there was no significant charge order in his samples. None.
“He came back and said, ‘The better samples didn’t show it,'” said Kyle Shen, the James A. Weeks Professor of Physical Sciences in the College of Arts and Sciences, who oversaw the project. “We were like, ‘Oh, that’s really weird. I don’t understand that.'”
Sometimes scientists get so stumped, they have no choice but to set aside their hypotheses, roll up their sleeves and put on their detective hats. After some extensive sleuthing, Parzyck, Shen and their collaborators realized that they had, in fact, done everything right.
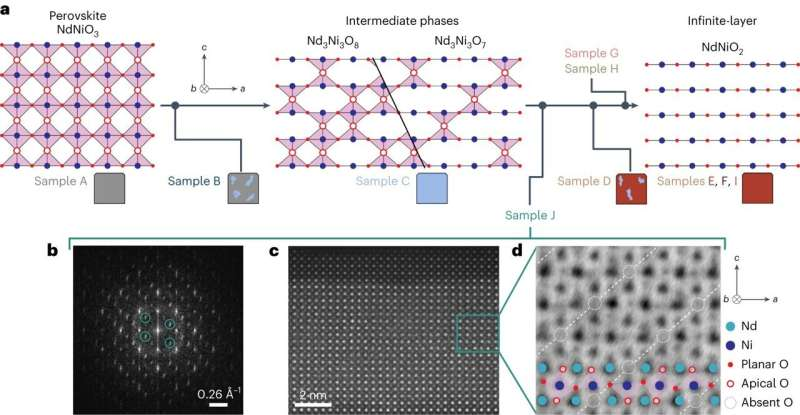
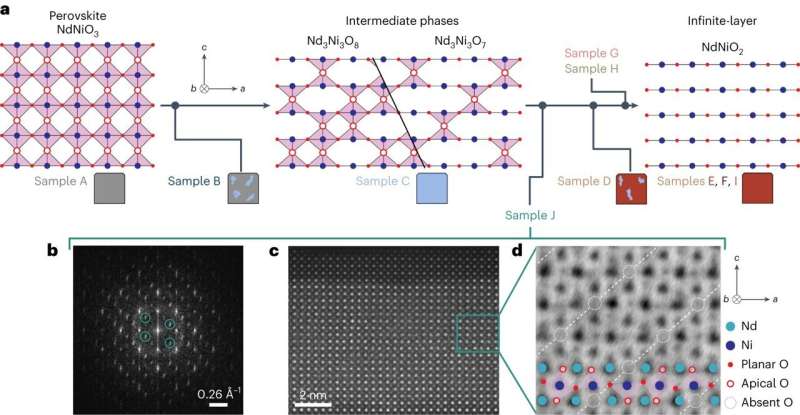
According to findings published Jan. 26 in Nature Materials, Parzyck’s new synthesis method produced nickelates that were so pure, they were free of the flaws that had tainted previous studies of nickelates. The charge order had never existed. They were chasing a phantom.
“Earlier reports said they see this charge ordering, but there were all these inconsistencies,” Shen said. “Chris developed a more controlled way of making these materials that effectively limits the number of defects. Excess oxygen atoms were masquerading as a signature of charge order.”
In recent years, nickelates have been the subject of considerable interest because they are newfound close cousins of the well-known “cuprates,” a family of copper oxide-based superconductors that can have high transition temperatures, upwards of 100 Kelvin, at which point electrical resistance vanishes, whereas for conventional superconductors, such as lead or niobium, their transitions are below 10 Kelvin. High-temperature superconductors are much easier to cool and thus are far more promising for potential future applications.
Ever since cuprates were first discovered in the late 1980s, scientists have sought similar superconducting families that might pinpoint the key qualities that enable high-temperature superconductivity.
“One obvious place to look is nickel, because nickel is right next to copper on the periodic table,” Shen said. “So people thought maybe we can do some material synthesis magic and make nickel-bearing compounds sort of like cuprates. That idea existed 30 years ago. The reason it took so long to realize is it turns out nickelate superconductors are really hard to make.”
Other researchers had synthesized nickelates—which are composed of nickel, oxygen and a rare earth element—by first growing a “precursor” material and then exposing that material to a source of hydrogen and heating them inside a sealed tube. Over the course of a day or so, the hydrogen pulls out roughly a third of the material’s oxygen molecules, which Shen compared to removing blocks in a game of Jenga.
“Synthesizing these materials is a bit of a nightmare,” he said.
Parzyck and Shen devised an alternative technique in which the oxygen is removed by a beam of atomic hydrogen, a process that is commonly used for cleaning semiconductor surfaces, but had never been used for materials synthesis. Atomic hydrogen reduction gives the researchers greater independent control of the amount of hydrogen being applied, in addition to variables such as time and pressure. The process can be completed in minutes, rather than hours or a day.
“Developing the reduction technique was a long and challenging process in-and-of-itself,” Parzyck said. “When I first started out, I tried to apply conditions like those used in traditional calcium hydride reduction—low temperatures for relatively long periods of time—but the sample quality was always low and not very consistent. It wasn’t until I decided to start fresh and go in a completely different direction—opting for higher temperatures for as short of a duration as possible—that I really found some success.”
After their synchrotron experiments failed to show the “resonant scattering peak” that should have signaled the presence of charge ordering, the researchers began varying the amount of oxygen they were stripping out.
“The real breakthrough came when we started measuring the samples which we purposefully prepared to have excess oxygen and saw a very strong, clear response—then we had a viable alternative explanation for the peak’s origin and finally knew we were going in the right direction,” Parzyck said.
To confirm their suspicions, they collaborated with the late Lena Kourkoutis, M.S. ’06, Ph.D. ’09, associate professor of applied and engineering physics, David Muller, the Samuel B. Eckert Professor of Engineering and their doctoral student Lopa Bhatt, who used electron microscopy to directly verify that trace amounts of oxygen in the samples were indeed causing the spurious charge-order signal.
Not only has the team identified a crucial difference between cuprate and nickelate superconductors; they now have a more reliable method for growing cleaner samples that can potentially be used for a wider variety of experiments, with a little less mystery.