by Yongji Qin
Hydrogen is widely recognized as a promising clean energy source, primarily due to its high energy density and the absence of carbon emissions during its utilization. This characteristic makes hydrogen an ideal candidate for addressing the growing energy demand and mitigating the environmental impact associated with the excessive use of non-renewable fossil fuels over the past decades.
To harness renewable energy from sources like solar, wind, and tidal power, a compelling strategy involves the conversion of this volatile energy into hydrogen. This approach not only aids in meeting the energy demand gap but also contributes to the overall sustainability of human society.
Presently, overall water splitting (OWS) is considered a viable method for hydrogen production. OWS, powered by renewable energy, facilitates the generation of hydrogen through the hydrogen evolution reaction (HER) on the cathode.
However, the Faradic efficiency of hydrogen production is impeded by the anodic oxygen evolution reaction (OER), which is characterized by sluggish kinetics and high thermodynamic potential.
Consequently, there is a pressing need for the development of advanced electrocatalysts for OER or other oxidation reactions with swift kinetics and low thermodynamic potentials.
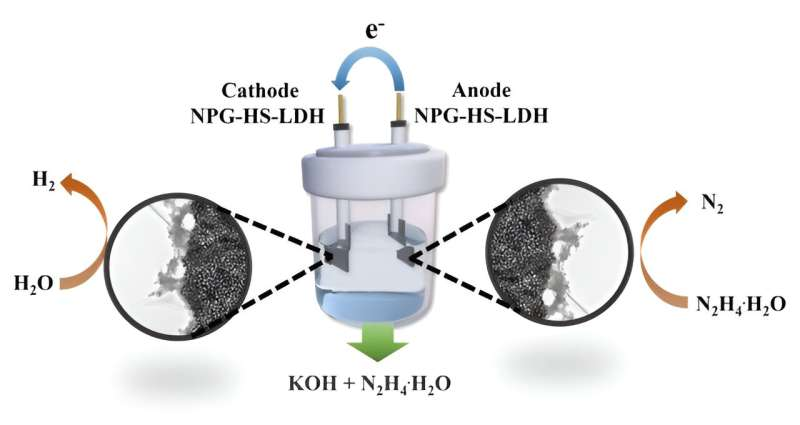
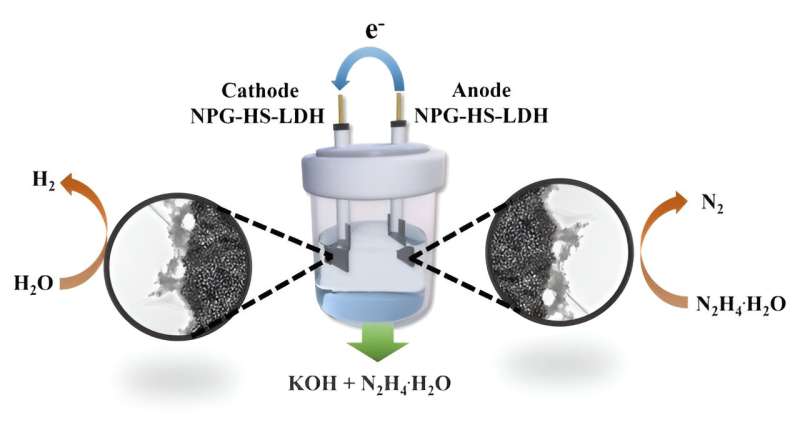
An alternative approach gaining traction is overall hydrazine splitting (OHzS) for hydrogen production, leveraging the anodic hydrazine oxidation reaction (HzOR). HzOR exhibits fewer electrons and faster kinetics compared to OER, making it a promising avenue. Nevertheless, a significant challenge remains in the synthesis of bifunctional electrocatalysts for both HER and HzOR with low overpotentials.
Recently, a research team in China introduced a novel solution in the form of a two-dimensional multifunctional layered double hydroxide derived from a metal-organic framework sheet precursor. This material is supported by nanoporous gold, providing high porosity. The study is published in the journal Frontiers of Chemical Science and Engineering.
Remarkably, this electrocatalyst demonstrates dual appealing activities for both HER and HzOR. In practical terms, the OHzS cell exhibits superior performance, requiring only a cell voltage of 0.984 V to deliver 10 mA∙cm-2, a notable improvement compared to the OWS system (1.849 V).
In addition, the electrolysis cell exhibits remarkable stability, operating continuously for more than 130 hours. This innovative approach not only enhances the efficiency of hydrogen production but also holds promise for a more sustainable and cleaner energy future.