by Ziyan Yang
Electrocatalytic reduction of carbon dioxide (CO2) is considered as an effective strategy for mitigating the energy crisis and the greenhouse effect. Among the multiple reduction products, CO is regarded as having the highest market value as it is a crucial feedstock for Fischer-Tropsch process which can synthesize high-value long-chain hydrocarbons.
Since the carbon dioxide reduction reaction (CO2RR) has complex intermediates and multiple proton-coupled electron transfer processes, improving the reaction activity and products selectivity remain two great challenges.
Single-atom catalysts (SACs) have the advantages of high atom utilization, tunable coordination structure and excellent catalytic performance. In addition, due to the special electronic structure of nickel metal, it is more likely to lose electrons to form empty outermost d-orbitals and exhibit high activity and selectivity for CO2RR to generate CO.
A team of scientists have summarized the considerable progress of Ni SACs in recent years. Their work is published in Industrial Chemistry & Materials.
“Designing novel catalysts to improve the activity and selectivity of CO2RR is crucial for conquering the problem of energy crisis and environmental pollution,” said Yuhang Li, a Professor at East China University of Science and Technology, China,
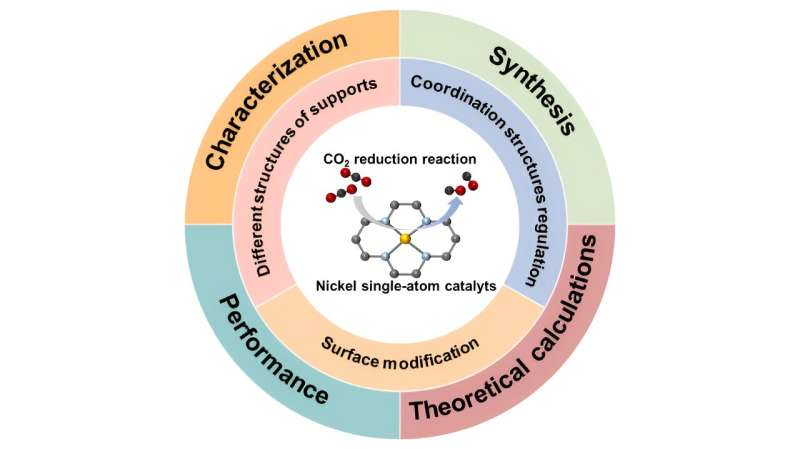
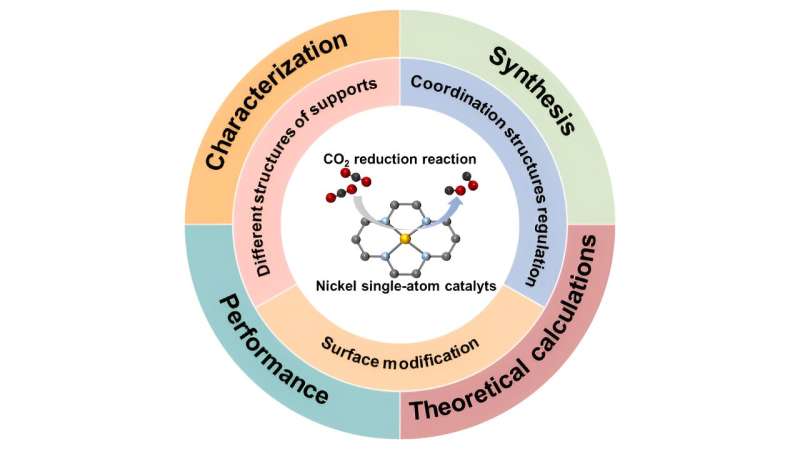
“In this mini-review, we introduced three strategies used to improve the catalytic performance of Ni SACs, including different structures of supports, coordination structure regulation, and surface modification. In the end, we also summarized the existing challenges of Ni SACs and provided an outlook on future development in this field.”
SACs downsize the active sites to atomic scale and therefore get extraordinary electronic structure, powerful metal-support interactions, low-coordinated metal atoms, and maximum atom utilization at the same time. Hence, the application of SACs in CO2RR could effectively control the distribution of products and alleviate the cost of products separation.
“Some research based on crystal-field theory has indicated that the d-orbital electronic configurations of central metals are significant to the selectivity and activity of CO2RR,” Li said.
“In the case of nickel as the central metal atom, it is more likely to form the vacant outermost d-orbital to facilitate the electron transfer between the C atom of CO2 and the Ni atom. Therefore, the absorbed CO2 molecules can be efficiently activated. Ni SACs can also minimize the reaction potential of CO2-CO conversion, which is of great importance to enhance the selectivity towards CO.”
“Ni SACs have achieved continuous progress in recent years. From a microscopic point of view, the design strategies include choosing different substrates, regulating the coordination structure and modifying the catalyst surface. The electronic structure of the active center is the most crucial factor affecting catalytic performance,” Li said.
There is still tremendous potential for Ni SACs in future designs and applications. Precise modulation of the microstructure provides more active sites and therefore further enhances the performance of Ni SACs. Optimization of the electrolytic cells and development of more types of electrolytes can expand the range of Ni SACs applications and enable large-scale commercialization in the future.
In addition, researchers think that developing more in-situ techniques to gain deeper insights into the relationship between material structure and properties can provide valuable guidance for designing higher-value Ni SACs.
“In this mini-review, our main goal is to provide readers with the current research progress in Ni SACs in CO2RR and to show our insights into the design and application of single-atom catalysts,” Li said.
The research team includes Ziyan Yang, Rongzhen Chen, Ling Zhang, Yuhang Li, and Chunzhong Li from East China University of Science and Technology.