Researchers have made a significant breakthrough in understanding how certain pathogens defend themselves against the host’s immune system.
This collaborative study, led by scientists from the University of Sheffield, as well as other British, Dutch and Chinese institutions focuses on the role of a group of enzymes known as zinc-dependent macrodomains (Zn-Macros) in reversing ADP-ribosylation, a vital cellular process.
This discovery could lead to innovative treatments to combat antimicrobial resistance, a growing global health threat. The work is published in the Journal of Biological Chemistry.
ADP-ribosylation is a reversible modification of proteins and DNA that regulates important cellular responses to stress. While this signaling mechanism is well-studied in higher eukaryotes, where it regulates responses to DNA damage, reactive oxygen species and infection, the importance of its role in microorganisms is also becoming increasingly evident, which includes the regulation of the host immune response, microbial immune evasion and adaptation to specific hosts.
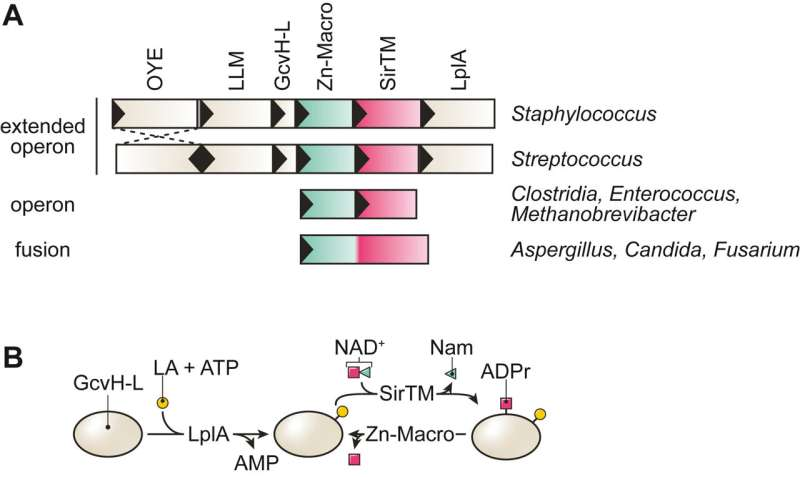
The research team used a combination of phylogenetic, biochemical, and structural approaches to investigate the function of Zn-Macros. These enzymes are found in some pathogenic microbes and are essential for removing ADP-ribosyl modifications, thereby helping the pathogens survive oxidative stress.
The study revealed that the catalytic activity of Zn-Macros is strictly dependent on a zinc ion within the active site of these enzymes. The researchers also identified structural features that contribute to substrate selectivity within different types of Zn-Macro enzymes, which may be exploited for the development of future therapies.
The findings have significant implications for the fight against bacterial and fungal infections that pose an increasing risk to human health, a problem that is exacerbated by the development of antimicrobial resistance and the emergence of multidrug-resistant strains. The World Health Organization has published lists of priority pathogens that pose the greatest risk, emphasizing the need for new antimicrobial strategies.
Addressing antimicrobial resistance will require a multifaceted strategy, including the discovery and characterization of new antimicrobial targets, along with assessing their potential for therapeutic use in innovative (co-)treatment approaches.
The authors of the study suggest that targeting the Zn-Macro pathway could reduce the virulence of major human pathogens, including Staphylococcus aureus and Streptococcus pyogenes. These pathogens rely on the crosstalk between lipoic acid metabolism and ADP-ribosylation signaling for their defense mechanisms. Disrupting this pathway could enhance the effectiveness of existing treatments and provide new therapeutic options.
The study’s findings represent a significant step forward in the fight against antimicrobial resistance and highlight the potential of Zn-Macros as therapeutic targets.
“Our findings uncover the evolutionary and molecular mechanisms behind ADP-ribosylation reversal by zinc-dependent macrodomains. By understanding this regulatory process, we can explore new avenues for drug development in diseases where ADP-ribosylation plays a critical role.
“I believe that further investigation into the physiological role of Zn-Macros could lead to the development of new antimicrobial therapies as these enzymes are primarily found in pathogenic microorganisms and have structurally distinct features that make them suitable for drug development,” says Dr. Antonio Ariza, university teaching associate.
More information: Antonio Ariza et al, Evolutionary and molecular basis of ADP-ribosylation reversal by zinc-dependent macrodomains, Journal of Biological Chemistry (2024). DOI: 10.1016/j.jbc.2024.107770
Journal information: Journal of Biological Chemistry