A research team affiliated with UNIST recently reported that the performance of electrodes for an alkaline hydrogen evolution reaction (HER) can be significantly improved even without expensive electrocatalysts and complicated processes by modifying them with superaerophobic polymeric hydrogels. This breakthrough has been led by Professor Jungki Ryu and his research team in the Department of Energy Engineering at UNIST.
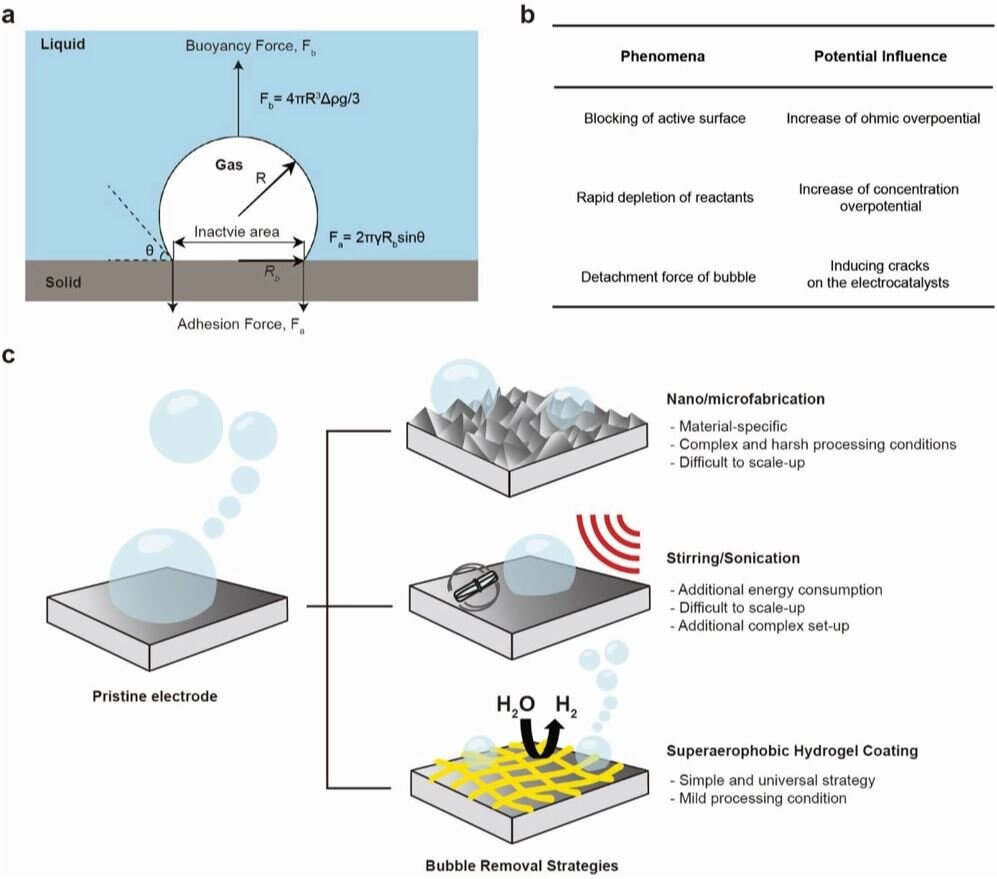
In this study, the research team reported a simple strategy to enhance the efficiency of electrochemical hydrogen production by imparting superaerophobicity to an underlying electrode with porous polymeric hydrogels. Superaerophobic hydrogels were readily coated on target substrates by cross-linking polyethyleneimine (PEI) via Schiff-base condensation reactions followed by freeze-drying, noted the research team.
As a result, they could readily control the pore size, porosity, and superaerophobicity of the hydrogel-coated electrodes by varying the concentrations of PEI upon cross-linking. Due to facile removal of as-generated hydrogen bubbles, the NF electrode modified with PEI hydrogel only outperformed those modified with expensive electrocatalysts especially at high current densities, according to the research team.
“We believe that our results can pave the way for the practical application of water electrolysis by providing insights into the design of electrodes and electrolyzers,” noted the research team.
This study has been published in Advanced Energy Materials.