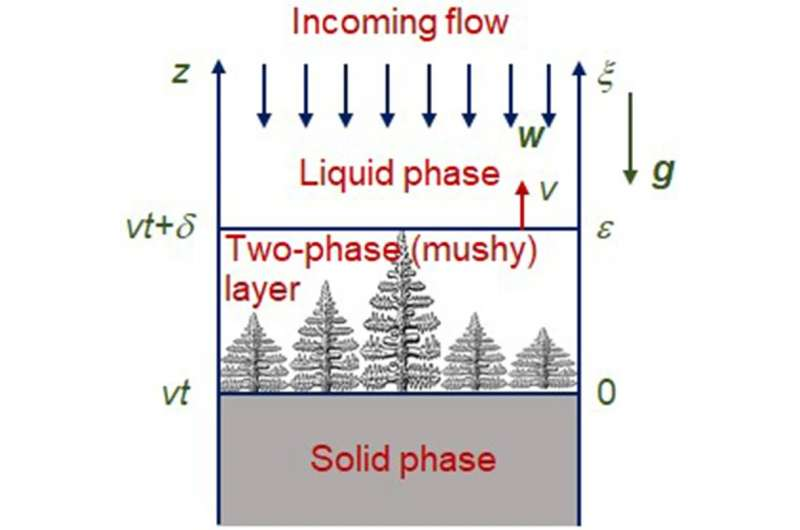
Physicists at the Ural Federal University (UrFU) have created a theory for the solidification of iron-nickel (Fe-Ni) alloy (invar). They determined that an important role in the technology of creating invar products, namely in the solidification process, is played by the oncoming flow: when an alloy cools, the liquid layer flows on top of the solidified layer. If this process is regulated, it is possible to control the characteristics of the alloys, obtain a more uniform structure, and improve the properties of the final product.
Nickel and iron alloys are used to create high-precision instruments, including clocks, seismic sensors, chip substrates, valves and engines in aircraft structures, and instruments for telescopes. The right calculations will help to create an alloy of the required structure, which will affect the quality of the finished products. A description of the model and the behavior of melts, as well as analytical calculations, was published in the journal Scientific Reports.
“Let me explain the work with the help of an analogy. When the water freezes, it pushes out all the dirt. So you can take a piece of ice in your mouth, it will be clean. Approximately the same happens with melts during cooling. Only they do not push out all the impurities, but some of them. Some of the impurities come out and some remain in the melt.”
“What remains in the melt fills the gaps between the crystals that solidify and the voids that remain. Thus, the alloys are heterogeneous: one tiny piece is enriched, while the neighboring piece is not. And it affects the properties of the finished product,” says Dmitri Alexandrov, Head of the Ural Federal University’s Laboratory of Multi-Scale Mathematical Modeling.
The main thing that scientists have shown is the processes in a two-phase layer: a layer in which both solid and liquid phases are located, inside it there is a transformation from a liquid state to a solid one.
“This layer completely changes the crystallization scenario. Thus, for example, the temperature at each point of this layer is lower than the crystallization temperature, and crystallites and dendrites release the heat of phase transformation and thus partially compensate for supercooling.”
“In addition, the growing solid phase displaces the dissolved impurity, which lowers the crystallization temperature. These processes lead to the formation of complex branched structures of the solid phase, the gaps between which are filled by a liquid with a higher concentration of impurities,” says Liubov Toropova, senior researcher at the Laboratory of Mathematical Modeling of Physical and Chemical Processes at UrFU.
The name “invar” comes from the word “unchanging,” as the alloy almost does not expand or contract when the temperature changes. The first invar alloy was discovered by Swiss scientist Charles Edouard Guillaume in 1896. In 1920 he received the Nobel Prize in Physics for it.
This alloy of nickel and iron is used when a serious dimensional stability of finished parts is required: in precision instruments, clocks, valves, etc. One of the first applications of invar is rods for pendulum clocks. At the time when the pendulum clock was invented, it was the most precise chronometer in the world. Today, invar is also used in astronomy, as components that support the size-sensitive optics of astronomical telescopes.
More information: Dmitri V. Alexandrov et al, The role of incoming flow on crystallization of undercooled liquids with a two-phase layer, Scientific Reports (2022). DOI: 10.1038/s41598-022-22786-w
Journal information: Scientific Reports
Provided by Ural Federal University