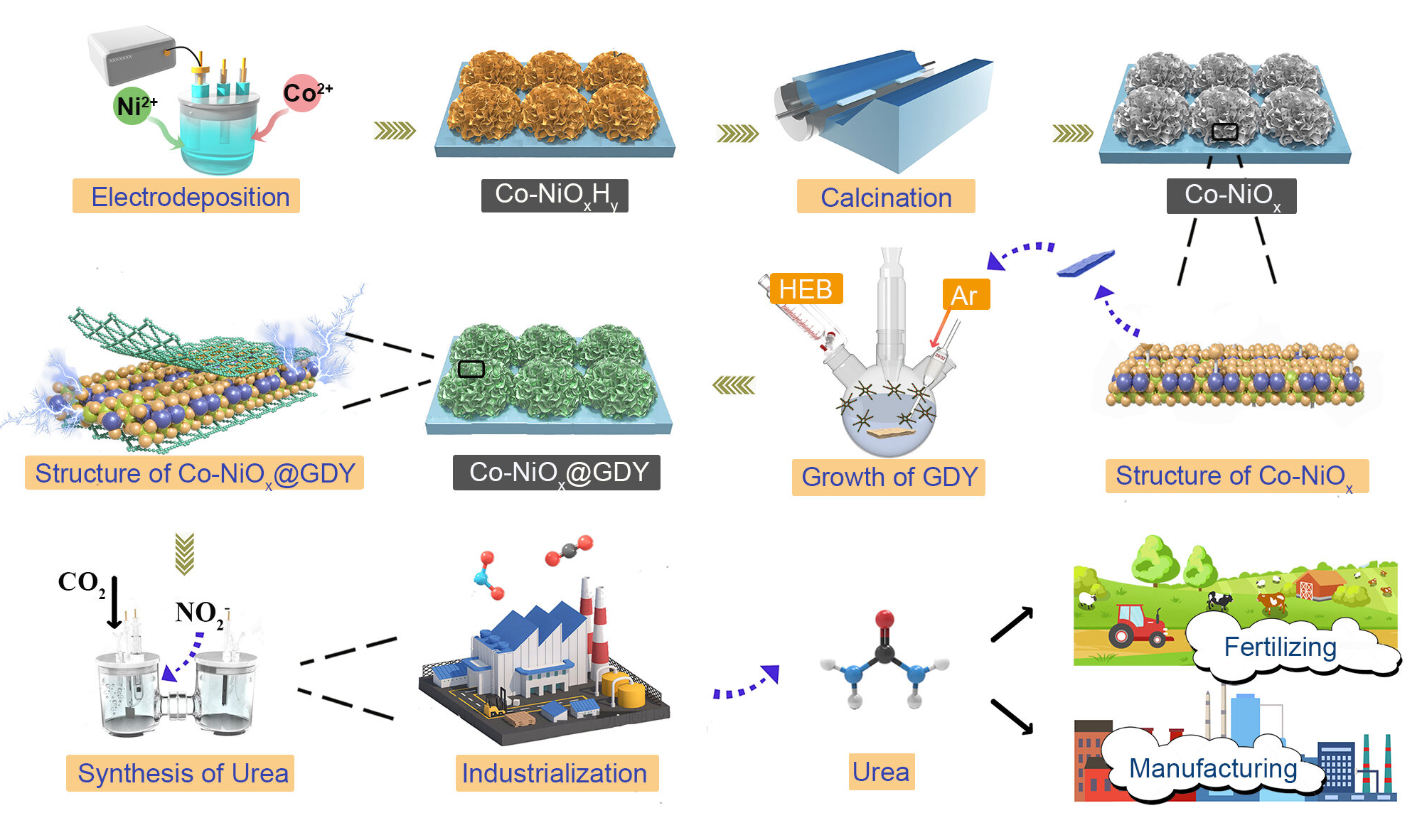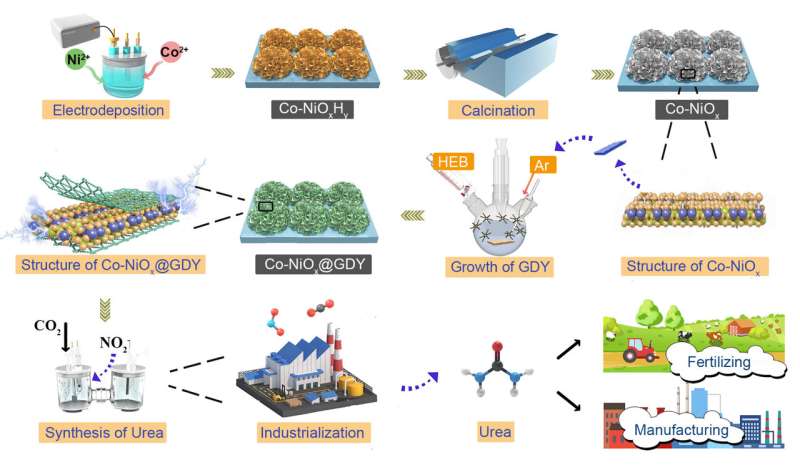
A major impediment to industrial urea synthesis is the lack of catalysts with high selectivity and activity. Prof. Yuliang Li (Institute of Chemistry, Chinese Academy of Sciences) and coworkers reported a new catalyst system suitable for the highly selective synthesis of industrial urea by in-situ growth of graphdiyne on the surface of cobalt-nickel mixed oxides.
The researchers found that such a catalyst is a multi-heterojunction interfacial structure resulting in the obvious incomplete charge transfer phenomenon between graphdiyne and metal oxide interface and multiple intermolecular interactions. These intrinsic characteristics are the origin of the high performance of the catalyst.
The team also demonstrated that the catalyst could effectively optimize the adsorption/desorption capacities of the intermediate and promote the direct C-N coupling by significantly suppressing by-product reactions toward the formation of H2, CO, N2, NH3.
The catalyst can selectively synthesize urea directly from nitrite and carbon dioxide in water at room temperature and pressure and exhibits record-high Faradaic Efficiency (FE) of 64.3%, nitrogen selectivity (Nurea-selectivity) of 86.0%, carbon selectivity (Curea-selectivity) of ~100%, as well as the urea yield rates of 913.2 μg h–1 mgcat–1 and remarkable long-term stability.
The work is published in the journal National Science Review.
More information: Danyan Zhang et al, Multi-heterointerfaces for selective and efficient urea production, National Science Review (2022). DOI: 10.1093/nsr/nwac209
Provided by Science China Press