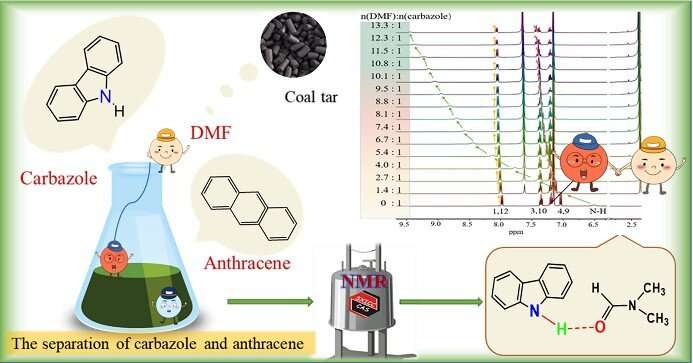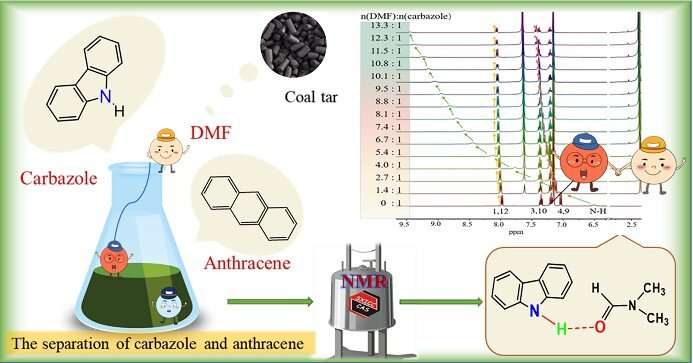
Coal tar, once considered waste, has become a huge treasure trove because hundreds of compounds can be isolated from it. Most of these compounds tend to be aromatic hydrocarbons, polycyclic aromatic hydrocarbons, and heterocyclic compounds.
Carbazole and anthracene, two aromatic hydrocarbon components contained in coal tar, are used as essential organic intermediates to synthesize various carbazole derivatives and anthraquinones. The effective separation of carbazole and anthracene takes advantage of their different solubility in solvents. In this process, solvent screening and performance optimization are essential, and their optimization mainly follows the principle of trial and error. Thus, it is necessary to use a versatile detection technique for understanding this separation process on the molecular level.
N,N-Dimethylformamide (DMF) has been developed as an efficient solvent for carbazole and anthracene separation due to the high solubility of carbazole in DMF; moreover, researchers have found that the separation of carbazole and anthracene may benefit from an intermolecular hydrogen bond between carbazole and DMF. However, there was no detailed study concerning the interaction mechanism between carbazole/anthracene and a solvent capable of hydrogen bonding. It is important to use a versatile detection technique for analyzing hydrogen bond interaction, and hence to explain the interaction mechanism between carbazole/anthracene and DMF via hydrogen bonding on the molecular level.
Recently, the group of Yan Qiao, a professor of the State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, CAS, studied the intermolecular interaction mechanism between DMF and carbazole/anthracene by various advanced liquid state NMR techniques. They observed that the N-H chemical shift of carbazole changed significantly in 1H NMR titration and VT-NMR experiments, indicating strong intermolecular hydrogen bonds between carbazole and DMF, which was further supported by the decrease in molecular self-diffusion coefficients (D) of both carbazole and DMF according to diffusion-ordered spectroscopy (DOSY) measurements.
Moreover, the Nuclear Overhauser Effect Spectroscopy (NOESY) experiment revealed that the distance between the aldehydic hydrogen of DMF and the N-H of carbazole was smaller than 5 Å. Accordingly, an intermolecular hydrogen bond between carbazole and DMF in the form of C=O···H-N was proposed.
“Solvent screening is still lacking theoretical guidance, with most work on the basis of ‘like dissolves like’ and lacks direct spectroscopic evidence,” Qiao said, “Our research helps researchers to understand the interaction mechanism between carbazole/anthracene and DMF in this process from the molecular and even atomic levels. It will also guide the further expansion of alternative solvent media and optimization of separation processes, and play an important role in promoting the development of coal tar separation industry.”
This research is published in the journal Industrial Chemistry & Materials.
More information: Hui Cao et al, Understanding the interaction mechanism of carbazole/anthracene with N,N-dimethylformamide: NMR study substantiated carbazole separation, Industrial Chemistry & Materials (2022). DOI: 10.1039/D2IM00020B
Provided by Institute of Process Engineering, Chinese Academy of Sciences