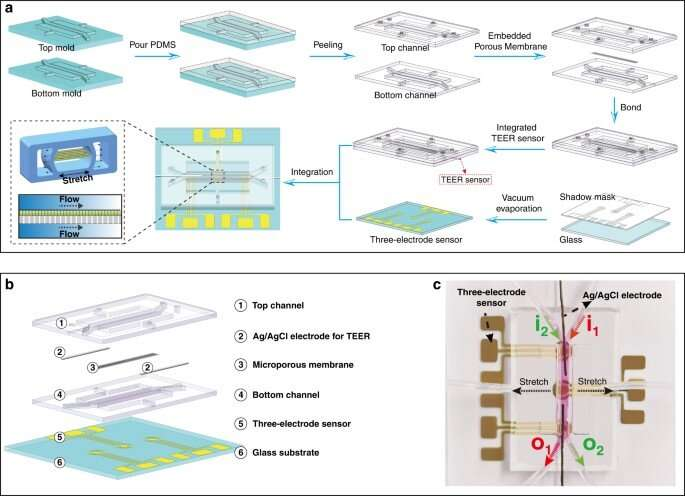
The transport of mercury ions across intestinal epithelial cells can be studied for toxicology assessments by using animal models and static cell cultures. However, the concepts do not reliably replicate conditions of the human gut microenvironment to monitor in situ cell physiology. As a result, the mechanism of mercury transport in the human intestine is still unknown.
In a new report now published in Nature Microsystems and Nanoengineering, Li Wang and a research team in mechanical engineering and regenerative medicine in China developed a gut-on-a-chip instrument integrated with transepithelial electrical resistance (TEER) sensors and electrochemical sensors.
They proposed to explore the dynamic concept to simulate the physical intestinal barrier and mirror biological transport and adsorption mechanisms of mercury ions. The scientists recreated the cellular microenvironment by applying fluid shear stress and cyclic mechanical strain.
Wang and the team studied mercury adsorption and the physical damage caused by the toxic element on epithelial cells via the performance of electrochemical sensors after exposing them to intestinal cells growing under diverse concentrations of mercury mixed in the cell culture medium. The team noted the corresponding expression and upregulation of Piezo1 and DMT1 (divalent metal transporter), both mechanosensory ion channels and iron transporters respectively on the cell surface.
Developing an intestinal model
Mercury ions are non-biodegradable and can accumulate in the body at low concentrations to cause damage to major organs. Toxic mercury ions can interact with antioxidant components, DNA repair enzymes and proteins at the subcellular level to disrupt cell homeostasis and produce disordered cellular structure and function.
While mercury adsorption occurs predominantly in the small intestine, high levels of mercury ingestion can cause internal bleeding and perforation in a short timeframe. The long-term intake of low concentrations of mercury ions can also lead to chronic intestinal diseases. Since the intestinal epithelium provides an initial barrier to limit the penetration of ingested mercury in the blood stream and reduce its harmful effects; a reasonable intestinal model is of great significance to explore transport mechanisms of mercury in the lab. Although animal models and static cell cultures are traditionally used to study intestinal adsorption and mercury transport, these models do not efficiently recapitulate the intestinal microenvironment to emulate the living intestine.

In this work, the researchers developed a gut-on-a-chip model integrated with label-free sensors, to non-invasively monitor changes in transepithelial electrical resistance (TEER) during cellular behavior of mercury absorption, in real time. The team identified key features of the gut via electrical measurements and immunohistochemistry studies, to assess the effect on mechanosensory ion channels.

Numerical analysis of the device
The research team developed a chip that showed mechanical behavior similar to the living intestine and introduced representative fluid flow and cyclic mechanical stretching. For instance, a shear stress approximating 0.02 dyne/cm2 produced a fluid flow required for intestinal epithelial morphogenesis. Based on additional calculations on the organ chip, the team obtained a flow rate of 160 μL/hour for the corresponding dimensions of shear stress.
They then simulated the tensile strain/stress dynamics via finite element analysis to understand the effects of the parameters on the physical properties of the instrument including its pore diameter and their impact on the cytodifferentiation by analyzing the catalytic activity of alkaline phosphatase; typically used as a marker of bone and liver damage. The team noted greater catalytic activity among cells under fluid flow and those undergoing mechanical strain on a chip after only seven days of cell culture.

The outcomes highlighted the biomimetic approach and the capacity for growth and differentiation of the cell monolayer under mechanical stimulation. The gut-on-a-chip instrument provided biomimetic intestinal villus-like structures to maintain the integrity of the tissue barrier and represented a key physiologically relevant human intestine.

Functional assays
The research team next exposed the cells to mercury under static culture conditions to understand the process of cell death. And noted an increase to the process when upon increasing the mercury concentration and culture time. They noted that the activity of lactate dehydrogenase (LDH) increased with time in the assays to show equal effects of the toxin across both cell cultures. However, the degree of injury between the two cultures differed. For instance, the expression of LDH was greater in the static culture compared to the dynamic cell cultures after mercury treatment.
The scientists used an electrochemical sensor array integrated into a gut-on-a-chip device to observe epithelial cell interactions with mercury. They explored the transport mechanism of the element relative to its absorption on epithelial cells and investigated the expression of key proteins such as Piezo1 and DMT1 relative to mechanosensory ion channels and active iron transporters. They studied the effects of different tensile strains on the cell barrier and noted that an increase in mechanical stimulation led to increased adsorption of mercury via intestinal epithelial cells, where the mechanosensory ion channels too showed a positive correlation.
Outlook
In this way, Li Wang and colleagues developed a gut-on-a-chip device integrated with transepithelial electrical resistor sensors and multiple electrochemical sensors to stimulate mercury transport in the human intestine in vitro. The chip dynamics emulated a physical intestinal barrier and microenvironment to observe the transport of mercury in real-time. The team noted the cell monolayer on the gut-on-a-chip to differentiate to form a complete cellular barrier via imaging and immunohistochemistry. The team intends to further understand additional mechanisms underlying human intestinal diseases using organ chips to promote personalized drug development.
More information: Li Wang et al, Gut-on-a-chip for exploring the transport mechanism of Hg(II), Microsystems & Nanoengineering (2023). DOI: 10.1038/s41378-022-00447-2
Sangeeta N Bhatia et al, Microfluidic organs-on-chips, Nature Biotechnology (2014). DOI: 10.1038/nbt.2989
Journal information: Nature Biotechnology
© 2023 Science X Network