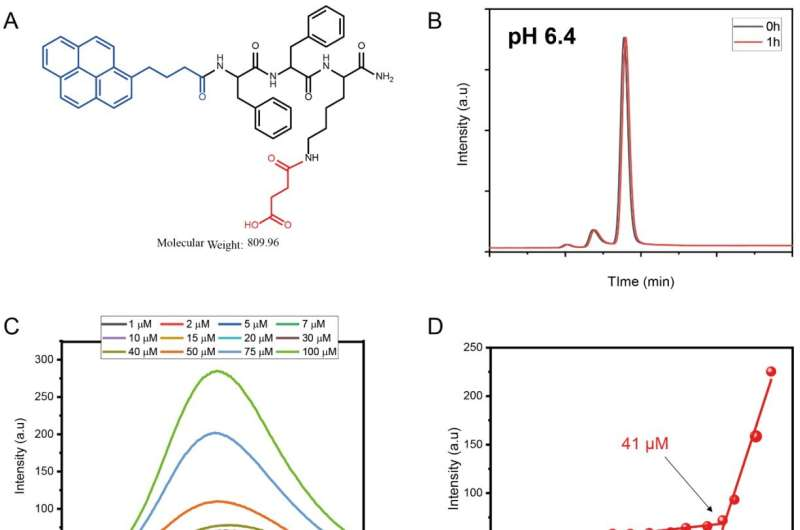
It is well known that cancerous tumor cells have an acidic pH microenvironment (pH 5.6 to 6.8). Using this unique feature, researchers have developed a new anticancer therapeutic agent that selectively kills cancer cells. This access allows detached malignant cells from the tumor to penetrate into cancer cells and induce mitochondrial dysfunction, thereby killing only cancer cells.
This breakthrough has been developed by Professor Ja-Hyoung Ryu and his research team in the Department of Chemistry at UNIST.
In the study, the research team developed transformable nano-assemblies of a mitochondria-targeting agent, Mito-SA, to achieve enhanced tumor selectivity. According to the research team, the new substance formed micelles containing a negative surface charge that selectively disassembled near the tumor cells into parental positively charged Mito-FF molecules, which induced apoptosis via self-assembly into nanofibers in the cancer cell mitochondria.
However, the entry of the Mito-SA micelle inside the normal cell is restricted due to the repulsion between the negatively charged micelle and the negatively charged plasma cell membrane, noted the research team. Furthermore, their findings also revealed that Mito-SA displayed an ideal tumor reduction ability and showed no side effect/damage to the normal tissue.
“Our work shows a strategy for the efficient delivery of positively charged, mitochondria-targeting agents by developing charge-shielded nano-assemblies that selectively disassemble in the tumoral environment,” said the research team.
Their findings have been published in Advanced Functional Materials.
More information: M. T. Jeena et al, Cancer‐Selective Supramolecular Chemotherapy by Disassembly‐Assembly Approach, Advanced Functional Materials (2022). DOI: 10.1002/adfm.202208098
Journal information: Advanced Functional Materials
Provided by Ulsan National Institute of Science and Technology