Stress isn’t just the psychological pressure you feel in response to a looming deadline at work. It is also a description of the physical forces pushing, pulling, or twisting an object, structure, or material. Examples of stress include gravity dragging downward on a bridge, wind blowing against the side of a building, or even a waistband drawn taut by a big meal.
With stress affecting literally everything made and used by people, often in damaging ways, it is important to identify when and where it is happening and the extent to which it is occurring. This is not always easy, though, because many materials show no obvious signs of being under stress.
Caltech’s Maxwell Robb, an assistant professor of chemistry, has been working to make stress easier to identify through the creation of polymers that change color when a force is applied to them. Now, in a paper titled “Mechanically gated formation of donor–acceptor Stenhouse adducts enabling mechanochemical multicolour soft lithography” and published in Nature Chemistry, Robb shows how his team created a new type of these polymers that can be made to change to almost any colors the user wants. This is in contrast to the polymers he had previously developed, which could only change to a single, predetermined color.
To understand the Robb group’s latest research, it is helpful to first know how previously developed color-changing plastics work. To create a plastic that changes color in response to stress, you need a type of molecule known as a mechanophore. There are many kinds of these mechanophores, with only some being color changing, but almost all work in essentially the same way. The molecules exist in two states, and when they are subjected to an external force, they undergo a chemical reaction that converts them from one state to another. In the case of the mechanophores Robb’s team works with, one state is colorless and the other is colored.
When mechanophores are incorporated into a plastic, they experience the force that is applied to the plastic and thus will change color, allowing the location of that stress to be visualized.
The team’s latest work is based on similar principles but with a twist. The mechanophore they have developed doesn’t change color directly with force but rather produces an intermediate compound that can be converted to myriad brightly colored dyes called donor–acceptor Stenhouse adducts (DASAs).
The colorless mechanophore molecules are also incorporated into plastic, but, in this case, they remain essentially colorless even after they are subjected to stress. Instead, the change in their molecular structure makes them able to undergo a secondary chemical reaction, a process referred to as mechanochemical gating. Once in that chemically receptive state, they are “developed” with a chemical bath that tacks another molecular piece onto their structure. These pieces cause the larger molecule to become brightly colored; the researcher can tune the color to any desired hue by changing the molecular fragment that is tacked on.
“The beauty of this is we have one central mechanically responsive molecule,” Robb says. “And that makes it a general chemical platform, because just by changing the identity of the chemical that you use in the second step, you can generate this extremely diverse library of dyes.”
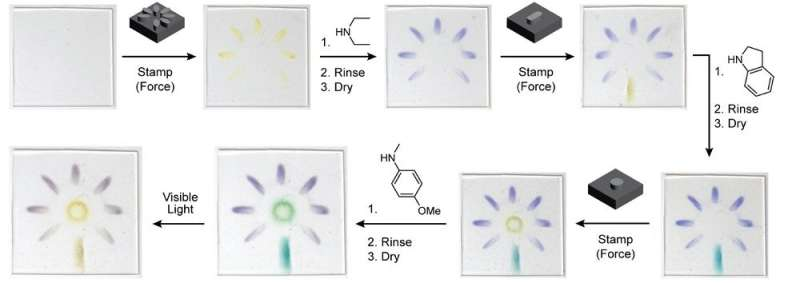
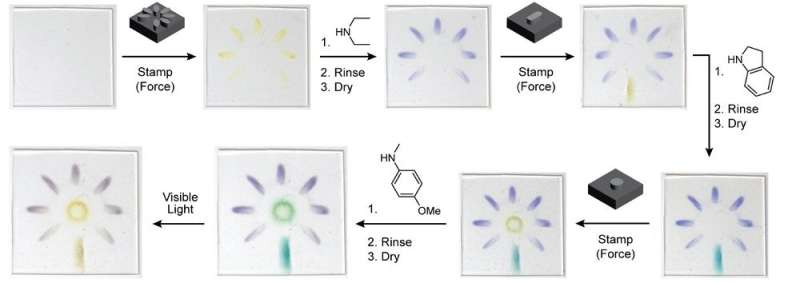
As a demonstration of the platform, researchers in Robb’s lab prepared a sheet of stretchy polymer containing their new mechanophore molecule and stamped it with a pattern that provided stress in specific areas. The material was then placed in a chemical bath that turned the pattern purple. The plastic was stamped a second time with a new pattern and developed with a different chemical that turned the newly stamped area blue. And finally, the plastic was stamped a third time and developed with a bath that turned the most recently stamped area green. Together, the three stamped patterns created a multicolor flower image within the plastic itself. And because DASAs also change their color with light, the image could be made to disappear using a flashlight.
Of course, the technology was not created just for the sake of printing simple pictures in plastics. Robb says the ability to leave chemically reactive imprints in three dimensions in polymer materials could also open the door to patterning other kinds of chemicals in three-dimensional space, such as proteins, which would have applications in tissue engineering. Additionally, he says that the lab is working to further take advantage of the time element associated with the photoresponse of DASAs, making the printing 4D.
“It’s actually really interesting for future directions of using this for things like encryption technologies,” he says.
More information: Anna C. Overholts et al, Mechanically gated formation of donor–acceptor Stenhouse adducts enabling mechanochemical multicolour soft lithography, Nature Chemistry (2023). DOI: 10.1038/s41557-022-01126-5
Journal information: Nature Chemistry
Provided by California Institute of Technology