Researchers from Japan have unlocked the potential of tannic acid and ultra-high molecular weight polyethylene oxide by using them to synthesize strong and smart supramolecular gels in a zero-waste process. These gels exhibit remarkable characteristics, such as high elongation, strong adhesion, resistance to swelling, shape memory, self-healing property, and biocompatibility.
Going forward, these innovative, zero-waste gels can have promising applications as advanced medical materials, promoting a sustainable approach to material science.
Recent advances in chemistry have allowed for the cost-effective synthesis of supramolecular materials with advanced properties. Due to their unique properties, such as toughness, elasticity, self-healing, biodegradability, and shape memory, these materials find diverse applications as advanced materials in various fields. However, their fabrication often involves time-consuming and complex processes, organic solvents, and waste production, resulting in low yields and high synthesis costs.
One promising option is to use tannic acid (TA), a biodegradable polyphenol that can form unique supermolecules by bonding strongly with other molecules. It is a safe, affordable, and environmentally sustainable material with multiple applications, such as those in the pharmaceutical industry.
In recent years, TA has been used for the synthesis of supramolecular gels using many types of polymers, including macromolecules like polyethylene oxide (PEO). However, while such studies have utilized relatively low-molecular-weight PEO for creating TA gels, ultra-high-molecular-weight (UHMW) PEO has been less commonly used.
Inspired by these studies and the ability of TA to form a viscous glue-like liquid on mixing it in water with PEG, a research team led by Associate Professor Taishi Higashi and graduate student Yuika Goto, from the Graduate School of Pharmaceutical Sciences, Kumamoto University, decided to develop hydrogels containing TA and PEO.
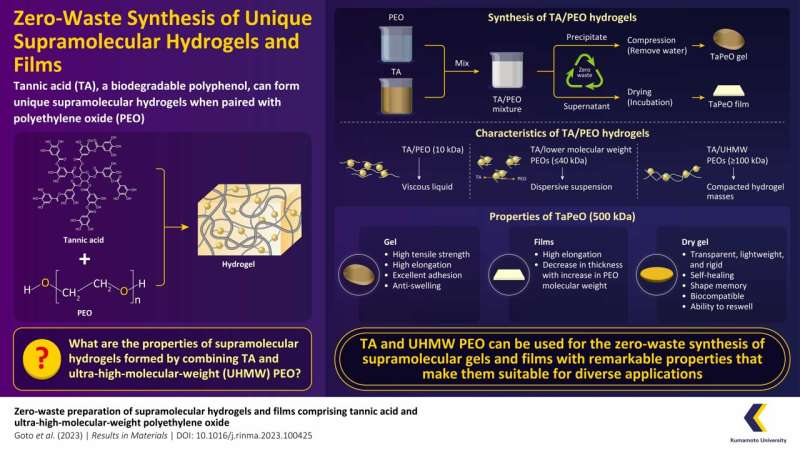
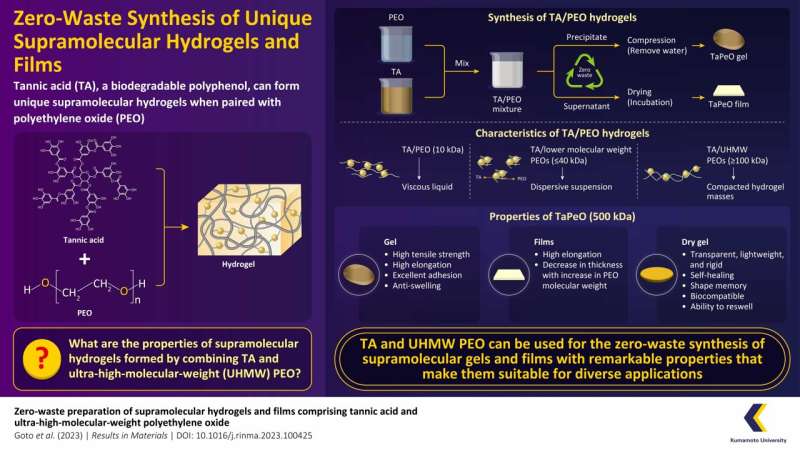
Their experiments have revealed that when TA and UHMW PEO are mixed together in water, a highly stretchable gel, named “TaPeO gel,” is formed via a zero-waste process. This study was made available online on in the journalResults in Materials in September 2023.
“We started by dissolving TA in water, and then PEO solutions with different molecular weights were mixed with TA at specific ratios to create TA/PEO gels. Following this, we used UHMW PEO with a molecular weight of 500 kDa to create TaPeO Gels. They were obtained by mixing TA and PEO solutions at a ratio of 1:2 (v/v) and compressing the precipitated gel,” says Associate Professor Higashi.
Named TaPeO Gel after the TA family, the mechanical properties of this gel were impressive, stretching remarkably and demonstrating a maximum elongation of up to 1,000% or more. It demonstrated excellent adhesion ability, as observed when the cut surfaces were touched, resulting in the reattachment of TaPeO Gel.
The maximum tensile strength and elongation of the reattached gel were comparable to the original gel after adhesion. Additionally, it had a water content of approximately 20% and low swelling ratios, ranging from 105% to 107%. It is interesting to note that the mechanical properties of TaPeO Gels varied with PEO molecular weight, with longer PEO chains leading to higher maximum tensile strengths.
The team further prepared dry TaPeO Gel by drying the wet gel at 40°C for eight days. They observed that upon drying, TaPeO Gel transformed into a transparent, lightweight, plastic-like rigid material with self-healing properties. They were also found to possess shape memory ability, observed by the gel’s ability to return to its original shape upon immersion in hot water after deformation.
The mechanical properties of dry TaPeO Gel were influenced by PEO molecular weight, with higher molecular weights leading to higher three-point bending flexural strengths. After immersion in water, the reswollen dry TaPeO Gel exhibited mechanical properties similar to those of the wet TaPeO Gel, indicating reversibility of gel properties.
To evaluate their biocompatibility, human cervical cancer cells were exposed to the TaPeO Gel. These cells showed almost 100% viability, indicating low cytotoxicity and good biocompatibility of the synthesized gel.
Moreover, during the preparation of the gel, the team encountered an excess liquid byproduct. Instead of discarding it, they opted to dry the supernatant of the TA/PEO mixture. The resulting TaPeO films, although initially brittle, exhibited excessive elongation (over 1,500%) after reabsorbing moisture, which was beyond the measurement scope of the testing machine used in this study.
The thickness of these films decreased with increasing PEO molecular weight, as higher molecular weight PEO led to more efficient entanglement with TA and lower concentrations in the supernatant. Associate Professor Higashi says, “These findings suggest that supramolecular films with high elongation can be prepared from the supernatant of TA/PEO mixtures, potentially offering zero-waste production of gels and films.”
This study provides valuable insights for the development of innovative supramolecular functional materials based on TA and UHMW PEO. These materials could hold great promise for various eco-friendly applications, on account of their exceptional properties.
Moreover, the zero-waste production process aligns well with the objective of environmental sustainability, making these materials highly desirable in the future.
More information: Yuika Goto et al, Zero-waste preparation of supramolecular hydrogels and films comprising tannic acid and ultra-high-molecular-weight polyethylene oxide, Results in Materials (2023). DOI: 10.1016/j.rinma.2023.100425
Provided by Kumamoto University