by Li Yuan, Chinese Academy of Sciences
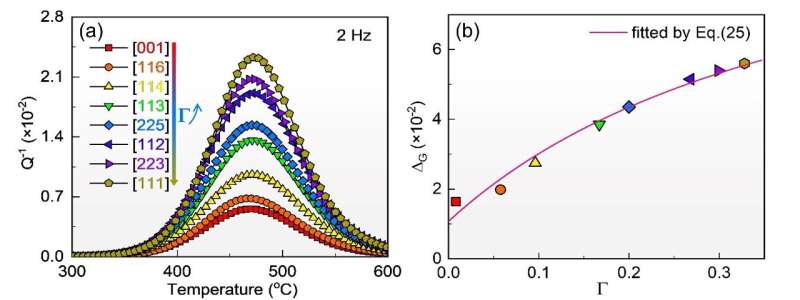
The stress-induced reorientation of low-symmetry defects due to substitution atom pairs can give rise to an internal friction peak called Zener relaxation. It is one of the most representative point defect relaxations in metal.
Recently, researchers from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences have revealed that Zener relaxation in the body-centered cubic (BCC) Fe-Ga single crystals originates primarily from the stress-induced reorientation of the second-nearest-neighbor Ga-Ga atom pairs, not the previously thought first-nearest-neighbor substitutional atom pairs. The study was published in Acta Materialia.
Fe-Ga alloys have great potential in the field of actuators, sensors, and micro-vibration suppression. However, their magnetostriction and damping capacity are closely related to the occupation of Ga atoms. The internal friction technique is sensitive to internal defect relaxation, so it is expected to solve the problem of evaluating Ga atom occupation and provide guidance for improving Fe-Ga alloy properties.
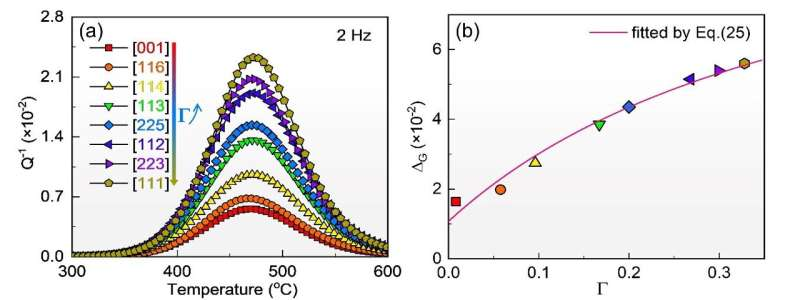
In this study, the team prepared large FeGa single crystals and FeGa binary single-crystal alloys with different orientation factors. A comparative study of the internal friction behavior of FeGa polycrystals and single crystals confirmed that the relaxation peak near 450°C originated from Zener relaxation behavior within the grains. It was observed that the net peak height of Zener relaxation increased with the orientation factor of single crystals.
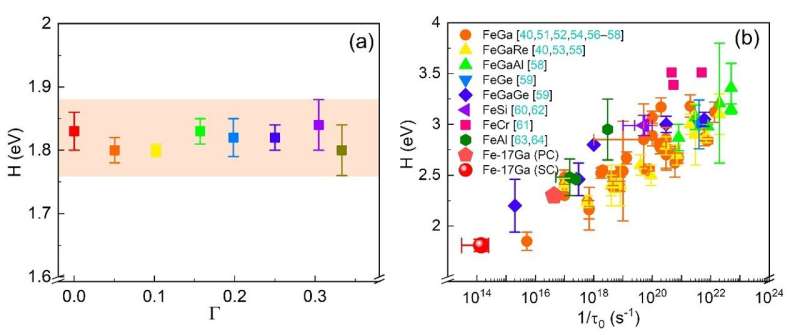
Further analysis revealed that the relaxation strengths of BCC cells with different atom-pair configurations varied. Trigonal and orthorhombic configurations exhibited decreased relaxation strengths with increased orientation factors, while tetragonal configuration showed the opposite trend. The relaxation activation energy for Fe-17at.% Ga single crystals was found to be around 1.8 eV, lower than that measured in polycrystalline materials.
Additionally, the researchers revealed a positive correlation between Zener relaxation strength and the magnetostriction coefficient through electronic structure and strain analysis.
More information: Meng Sun et al, Tetragonal dipole dominated Zener relaxation in BCC-structured Fe-17at.%Ga single crystals, Acta Materialia (2023). DOI: 10.1016/j.actamat.2023.119245
Journal information: Acta Materialia
Provided by Chinese Academy of Sciences