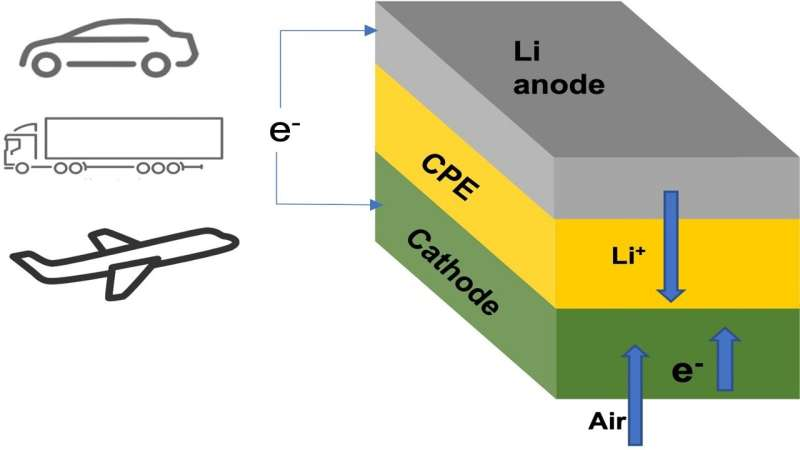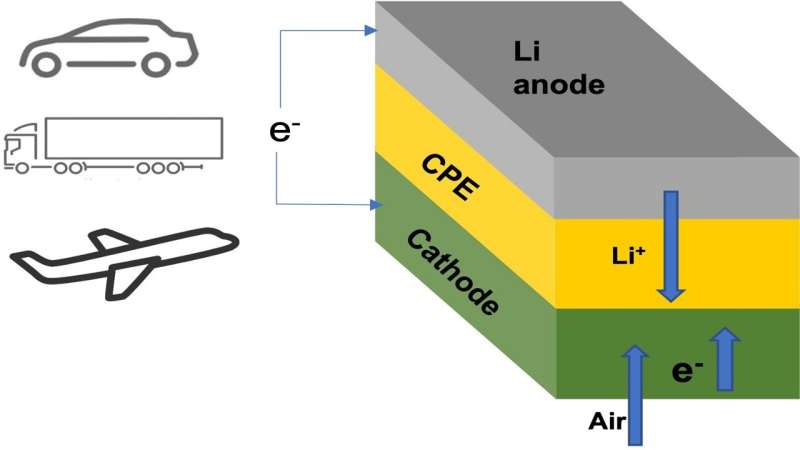
Many owners of electric cars have wished for a battery pack that could power their vehicle for more than a thousand miles on a single charge. Researchers at the Illinois Institute of Technology (IIT) and U.S. Department of Energy’s (DOE) Argonne National Laboratory have developed a lithium-air battery that could make that dream a reality. The team’s new battery design could also one day power domestic airplanes and long-haul trucks.
The main new component in this lithium-air battery is a solid electrolyte instead of the usual liquid variety. Batteries with solid electrolytes are not subject to the safety issue with the liquid electrolytes used in lithium-ion and other battery types, which can overheat and catch fire.
More importantly, the team’s battery chemistry with the solid electrolyte can potentially boost the energy density by as much as four times above batteries”>lithium-ion batteries, which translates into longer driving range.
“For over a decade, scientists at Argonne and elsewhere have been working overtime to develop a lithium battery that makes use of the oxygen in air,” said Larry Curtiss, an Argonne Distinguished Fellow. “The lithium-air battery has the highest projected energy density of any battery technology being considered for the next generation of batteries beyond lithium-ion.”
In past lithium-air designs, the lithium in a lithium metal anode moves through a liquid electrolyte to combine with oxygen during the discharge, yielding lithium peroxide (Li2O2) or superoxide (LiO2) at the cathode. The lithium peroxide or superoxide is then broken back down into its lithium and oxygen components during the charge. This chemical sequence stores and releases energy on demand.
The team’s new solid electrolyte is composed of a ceramic polymer material made from relatively inexpensive elements in nanoparticle form. This new solid enables chemical reactions that produce lithium oxide (Li2O) on discharge.
“The chemical reaction for lithium superoxide or peroxide only involves one or two electrons stored per oxygen molecule, whereas that for lithium oxide involves four electrons,” said Argonne chemist Rachid Amine. More electrons stored means higher energy density.
The team’s lithium-air design is the first lithium-air battery that has achieved a four-electron reaction at room temperature. It also operates with oxygen supplied by air from the surrounding environment. The capability to run with air avoids the need for oxygen tanks to operate, a problem with earlier designs.
The team employed many different techniques to establish that a four-electron reaction was actually taking place. One key technique was transmission electron microscopy (TEM) of the discharge products on the cathode surface, which was carried out at Argonne’s Center for Nanoscale Materials, a DOE Office of Science user facility. The TEM images provided valuable insight into the four-electron discharge mechanism.
Past lithium-air test cells suffered from very short cycle lives. The team established that this shortcoming is not the case for their new battery design by building and operating a test cell for 1000 cycles, demonstrating its stability over repeated charge and discharge.
“With further development, we expect our new design for the lithium-air battery to also reach a record energy density of 1200 watt-hours per kilogram,” said Curtiss. “That is nearly four times better than lithium-ion batteries.”
This research was published in a recent issue of Science. Argonne authors include Larry Curtiss, Rachid Amine, Lei Yu, Jianguo Wen, Tongchao Liu, Hsien-Hau Wang, Paul C. Redfern, Christopher Johnson and Khalil Amine. Authors from IIT include Mohammad Asadi, Mohammadreza Esmaeilirad and Ahmad Mosen Harzandi. And Authors from the University of Illinois Chicago include Reza Shahbazian-Yassar, Mahmoud Tamadoni Saray, Nannan Shan and Anh Ngo.
More information: Alireza Kondori et al, A room temperature rechargeable Li 2 O-based lithium-air battery enabled by a solid electrolyte, Science (2023). DOI: 10.1126/science.abq1347
Journal information: Science
Provided by Argonne National Laboratory