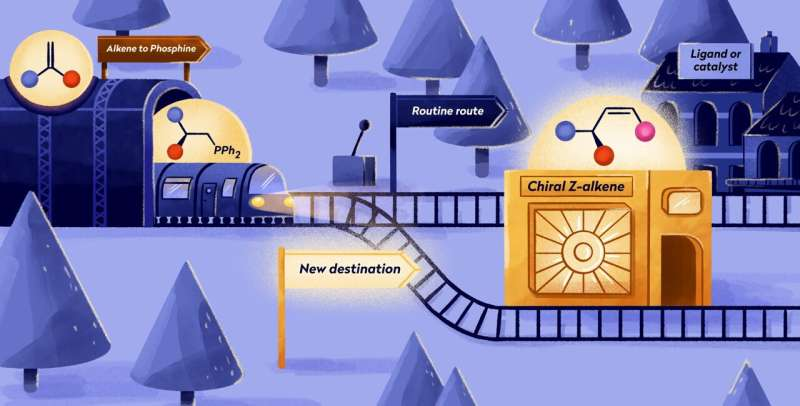
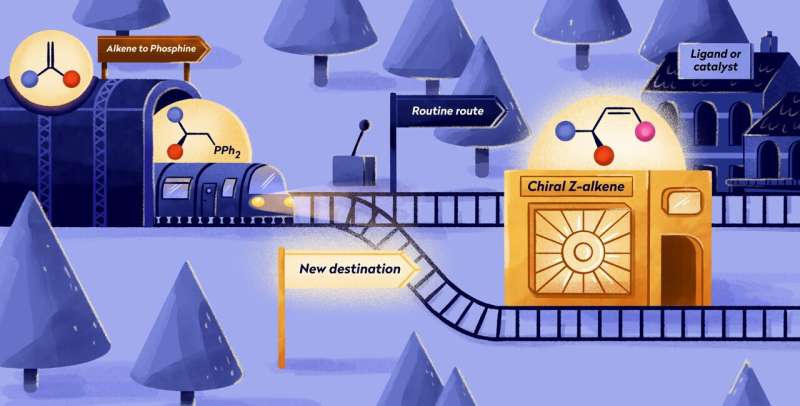
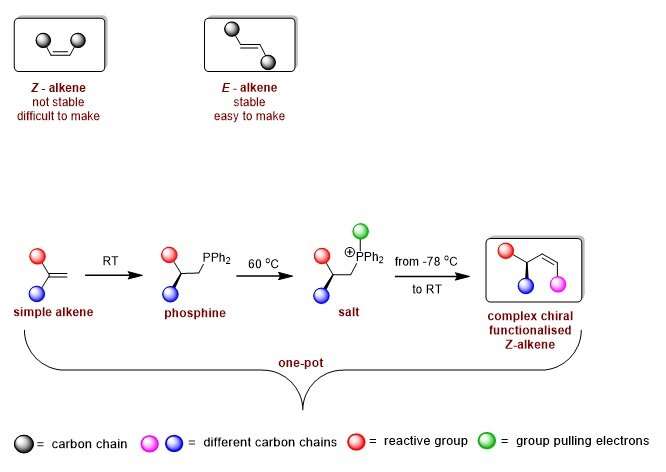
Ever considered the carbon footprint of manufacturing your favorite shirt?
The average cotton shirt produces 2.1 kilograms of carbon dioxide—but a polyester shirt produces over twice as much (5.5 kilograms). It might come as no surprise that the fashion industry is responsible for around 5% of global CO₂ emissions.
Some natural fibers can also take a heavy toll on the environment. Last week, for example, an ABC investigation revealed hundreds of hectares of the Northern Territory’s pristine tropical savanna had been cleared to make way for cotton farms, sometimes without permit.
So are there more sustainable textiles we should be producing and purchasing instead?
Research, including our own ongoing research, points to certain “non-traditional fibers” as new green alternatives. These include fibers produced from wastes—think coffee waste and recycled plastic bottles—as well as seaweed, orange, lotus, corn and mushroom.
Brands such as Patagonia, Mud Jeans, Ninety Percent, Plant Faced Clothing and Afends are among the brands leading the way in incorporating sustainable fibers into their products. But the true turning point will likely come when more of the biggest names in fashion get involved, and it’s high time they invest.
The problems with traditional fibers
There are two types of traditional fibers: natural and synthetic. Natural fibers, such as cotton and flax, have certain advantages over synthetic fibers which are derived from oil and gas.
When sustainability is considered, natural fibers are preferred over the synthetic fibers due to, for instance, their ability to biodegrade and their availability in the environment.
However, some natural fibers (particularly cotton) need a lot of fresh water and chemicals that are toxic to the environment for harvesting. For example, it takes 10,000 liters of water on average to grow just 1 kilogram of cotton.
In comparison, synthetic fibers consume a significantly lower amount of water (about one hundredth), but a significantly higher amount of energy.
Petrochemical fibers made from fossil fuels—such as polyester, nylon and acrylic—are the backbone of fast fashion. Yet another big problem with such products is that they don’t easily decompose.
As they slowly break down, petrochemical fibers release microplastics. These not only contaminate the environment, but also enter the food chain and pose health risks to animals and humans.
You may have also come across blended fabrics, which are produced with a combination of two or more types of fibers. But these pose challenges in sorting and recycling, as it’s not always possible or easy to recover different fibers when they’re combined.
Non-traditional fibers: a potential game changer
Amid the overconsumption of traditional fibers, several global fashion brands have started to adopt new fibers derived from seaweed, corn, and mushroom. This includes Stella McCartney, Balenciaga, Patagonia, and Algiknit.
Other emerging natural fibers include lotus, pineapple and banana fibers. Lotus fibers are extracted from the plant stem, banana fibers are extracted from the petiole (the stalk that connects the leaf and stem), and pineapple fibers are extracted from pineapple leaves.
The process of extracting fibers from wastes such as orange peels, coffee grounds, and even from the protein of waste milk, has also been well researched, and clothes have been successfully manufactured from these materials.
All these examples of non-traditional fibers are free from many of the problems mentioned earlier, such as heavy resource consumption (particularly fresh water), use of toxic chemicals, and the use of large amounts of energy (for synthetic fibers).
Further, these fibers are biodegradable at their end of life and don’t release microplastics when you wash them.
Meanwhile, there has been tremendous growth in the use of recycled synthetic fibers, which reduces the use of virgin materials, energy and chemical consumption. Recycling plastics such as drink bottles to make clothing is also becoming more common. Such innovations can help lower our dependency on raw materials and mitigate plastic pollution.
What’s more, the selection of appropriate color combinations during recycling and processing for fabrics can avoid the need for dyeing.
What now?
Fashion companies can reduce the load on the environment through seriously investing in producing sustainable fibers and fabrics. Many are still in research stage or not receiving wider commercial applications.
Fashion manufacturers, large fashion brands and retailers need to invest in the research and development to scale-up production of these fibers. And machine manufacturers also need to develop technologies for large-scale harvesting and manufacturing raw materials, such as sustainable fiber and yarn.
At the same time, you, as a consumer, have an important role to play by demanding information about products and holding brands accountable.
Provided by The Conversation
This article is republished from The Conversation under a Creative Commons license. Read the original article.