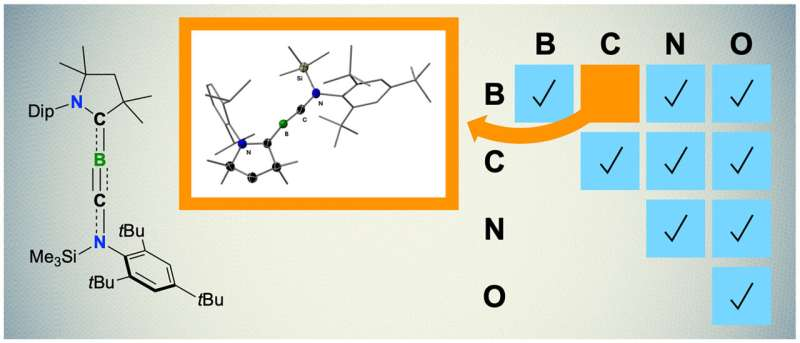
Boron, carbon, nitrogen and oxygen: these four elements can form chemical triple bonds with each other due to their similar electronic properties. Examples of this are the gas carbon monoxide, which consists of one carbon and one oxygen atom, or the nitrogen gas in the Earth’s atmosphere with its two nitrogen atoms.
Chemistry recognizes triple bonds between all possible combinations of the four elements—but not between boron and carbon. This is astonishing because there have long been stable double bonds between boron and carbon. In addition, many molecules are known in which triple bonds exist between two carbon atoms or between two boron atoms.
Chemists at Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, have now closed this gap: A team led by boron expert Professor Holger Braunschweig has succeeded for the first time in synthesizing a molecule with a boron-carbon triple bond, a so-called boryne, which exists as an orange solid at room temperature.
The scientists characterized the new molecule and also carried out initial reactivity studies. They present the results in the journal Nature Synthesis.
Boron atom in an uncomfortable situation
In the novel molecule, the boron atom is in a linear arrangement with carbon atoms. “In combination with the triple bond, this is about as uncomfortable as it gets for boron, requiring very special conditions,” says Dr. Rian Dewhurst, co-author of the study. This is why it has taken so long to synthesize such a triple bond for the first time.
What interests the Würzburg chemists about the new molecule: “Compounds in which individual atoms feel ‘uncomfortable’ often show a very interesting reactivity,” explains Maximilian Michel, the doctoral student who made the molecule in the laboratory.
It is precisely this reactivity that the team’s further work is now focusing on. Ultimately, this may result in innovative tools for chemical syntheses. The findings could also be helpful for a better understanding of chemical bonds and structures.
“Another benefit that is often overlooked: Basic research like ours inspires other researchers to put their efforts and imagination into synthesizing compounds that might seem improbable,” says Dewhurst. “World-changing advances often emerge from these kinds of crazy ideas.”
Teflon, for example, was discovered during research originally aimed at developing new refrigerants, while the well-known product superglue emerged by chance during attempts to produce transparent plastics.
More information: Maximilian Michel et al, The synthesis of a neutral boryne, Nature Synthesis (2025). DOI: 10.1038/s44160-025-00763-1. www.nature.com/articles/s44160-025-00763-1
Journal information: Nature Synthesis
Provided by University of Würzburg